Abstract
Single-phase SrGdxFe12−xO19 (x = 0.00–0.05) nanosized M-type hexagonal ferrites (NHFs) were prepared via citrate sol–gel route. The formation of Gd substituted Sr-hexaferrites has been confirmed by XRD, SEM, TEM and EDX for all substitutions. The X-ray powder patterns revealed the hexagonal crystal structure of all products. The electrical and dielectric properties of SrGdxFe12−xO19 NHFs were investigated extensively with an impedance spectroscopy up to 3.0 MHz from 20 to 120 °C. Both electrical and dielectric components including ac/dc conductivity, dielectric constant, dielectric loss and lossy tangent were evaluated for measurement temperatures up to 120 °C. It has been indicated that ac conductivity generally complies with power law rules, mainly dependent on Gd3+-ion substitution ratios. The impedance analysis showed that due to the influence of various Gd3+-ion substitution ratios in the NHFs, the conduction mechanisms can mainly be attributed to the grain-grain boundaries. The dielectric constant of SrGdxFe12−xO19 NHFs owns a normal dielectric distribution with the frequency, largely due to varying substitution ratios. In other words, the variation of Gd3+-ion substitution can be used to modify the conduction mechanism of NHFs. Therefore, the observed change in dielectric properties as a function of frequency can be clarified on a phenomenological basis by Koop’s model of the electrical conduction mechanism in most composite ferrites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hexagonal ferrites, also known as hexaferrites, are magnetic iron oxides with a hexagonal structure. They are formed by iron, oxygen and one or more other elements, which could be barium, strontium, cobalt or a combination of these [1]. There are structurally six types of hexaferrites (M, W, X, Y. Z U) which have widespread applications as Permanent magnets, Electrical and microwave devices, Data storage and recording media, RAM (random access memory), microwave/EMI (electromagnetic interference) wave absorption, Magnetoelectric (ME) and multiferroic (MF) applications, millimeter wave imaging, millimeter wave imaging, etc. [1]. Owing to their very unique physical properties (higher values of saturation magnetization, high electrical resistivity, high permittivity, high permeability, their tunable values of coercivity and low losses), M-type hexaferrites are suitable materials for permanent magnet applications and high-density recording media, etc. The room-temperature MF properties enable M-type hexaferrites to be employed in electromagnetic absorption in microwave region, high frequency applications, etc. [2,3,4]. The electrical properties of hexaferrites depend on both the type of hexaferrite and substituted element. All these properties can be tuned by the cationic replacements at Sr2+/Ba2+ and/or Fe3+ sites according to the requirements [5]. The rare earth ion (M3+) substituted hexaferrites, due to their tuned dielectric properties, can be considered for specific device applications [6, 7]. We can encounter different studies regarding the substitution of hexaferrites by M3+ ions in the literature. For example, magnetic properties and hyperfine interactions (Mössbauer study) of gallium-doped M-type barium hexaferrites was studied by Trukhanov et al. [8]. The polarization origin and iron positions in indium-doped barium hexaferrites was examined in a study by Trukhanov et al. [9]. The effect of Nb substitution on magnetic properties of BaFe12O19 nanosized M-type hexagonal ferrites was studied by Almessiere et al. [10]. The effect of magnetic, non-magnetic and dielectric ions substitution on magnetic and microwave properties of BaFe12O19 was studied by Sozeri et al. [11]. Baykal et al. studied the effect Pb substitution on microwave properties of Ba- and Sr-hexaferrite nanoparticles as high-quality microwave absorbers [12]. The magnetic and dielectric properties of bicomponent ceramics [(BaFe11.9Al0.1O19)1-x − (BaTiO3)x] containing the M-type crystal structure hexaferrite was studied by Trukhanov et al. [13]. The recent antenna application of M-type hexaferrites was explored by Darwish et al. [14]. In another study, Nemreva et al. showed the presence of three oxidation states of manganese in the Ba-hexaferrite (BaFe12−xMnxO19) [15].
Apart from M-type hexaferrites, other types of hexaferrites have been also intensively investigated. For instance, Häßner et al. synthesized a new hexaferrite with the composition (Ba, Pb) (Fe, Ti)9O15 by lead (II) oxide flux, which led to the incorporation of Pb2+ into the structure on the barium position [16]. The product showed ferrimagnetic behaviour with a Curie temperature of Tc = 309.3 °C and a strong indication for a magnetic phase transformation to ferromagnetic state at high magnetic field strengths and at temperatures below − 123 °C [16]. Nowadays, exchanged coupled hard (M-type hexaferrite)/soft (M-type hexaferrite) magnetic nanocomposites are promising a new filed of application [17,18,19]. In addition to their magnetic behaviour, hexagonal ferrites also exhibit interesting dielectric properties. Due to their interesting dielectric properties, they find large scale applications from microwave to radio frequency devices. The electrical properties of hexaferrites are highly sensitive to the type and element of substitution. By adding different cations and varying cations distribution between interstitial sites, the dielectric properties of the hexaferrites can be engineered for specific device applications [7, 20]. The doping of lanthanide (Ln3+) on strontium hexaferrite in very small amounts leads to significant improvements in their properties [21]. Furthermore, the Ln3+ dopant is not only useful for tuning the hexaferrites internal characteristics, but also can contribute to controlling the grain size at a higher temperature [22]. It has been reported [23] that the rare earth ions can modify the physical properties due to the larger ionic radius, which resides at B sites.
The aim of the current study is to prepare Gd3+-substituted M-type Sr-nanosized M-type hexagonal ferrites with the sol–gel auto-combustion method and investigate their structural, electrical and dielectric properties in detail.
2 Experimental
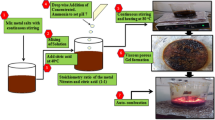
For the citrate sol–gel synthesis of SrGdxFe12−xO10 (x = 0.00–0.05) NHFs, Fe(NO3)3.6H2O (98%, Merck), Sr(NO3)2, (98%, Merck) and GdCl3.6H2O (97%, Merck) along with citric acid have been used. The certain amounts of metal nitrates and citric acid were dissolved in separate beakers containing 25 mL DI water under continuous stirring followed by combining both solutions to form the reaction mixture, whose pH was arranged to 7 by a concentrated ammonia solution. Afterwards, the final solution was heated up to 150 °C and then to 320 °C to have a viscous solution and finally a gel forming NHFs precursor. The solid product was obtained after being calcinated at 1100 °C for 6 h.
The structural, morphological analyses and conductivity and dielectric measurements were done by Rigaku Benchtop Miniflex X-ray diffractometer (Cu Kα radiation), FEI Titan ST scanning electron microscope (SEM, with EDX), FEI, Morgagni 268 Transmission electron microscopy and Novo-control Alpha-N high-resolution dielectric–impedance analyzer, respectively.
3 Results and discussion
3.1 Microstructure and morphology
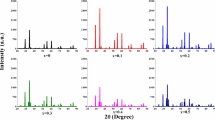
The structures of SrGdxFe12−xO19 (x = 0.00–0.05) NHFs were investigated by analysis of XRD powder patterns (Fig. 1). All patterns showed pure phase M-type Sr-hexaferrite crystal structure without occurrence of impurities. As it can be seen from Fig. 1, all peaks perfectly matched with the file PDF-33-1340 in ICDD, confirming the formation of the hexagonal crystal structure. The average crystallite size (DXRD) was calculated from (114) diffraction peak by applying a well-known Scherrer’s formula and found to be in the range of 34–50 nm. All structural parameters were given in Table 1.
Figure 2 exhibited the SEM images of SrGdxFe12−xO19 (x = 0.00–0.05) NHFs. All samples displayed highly agglomerated hexagonal platelet particles. These hexagonal platelets are nano-scale in thickness and micro-scale in diameter (the edge length in the range of 650–850 nm and the thickness of the platelet is about 35–60 nm). Moreover, the structure and morphology were confirmed via TEM and SAED images as seen in Fig. 2. The composition content of SrGdxFe12−xO19 (x = 0.03) NHFs was analyzed through EDX and it was found that all elements matched well with their chemical formula as seen in Fig. 3.
3.2 Electrical and dielectric properties
The permittivity of a variety of ferrite materials is used to explain a variety of physical phenomena. Electronic polarization, atomic polarization, dipolar relaxation and ionic conduction are the main contributors to the permittivity of a dielectric material in general. In this study, dielectric losses are much more dominant in ionic conductivity at frequencies under the microwave region [24]. The electrical and dielectric properties of SrGdxFe12−xO19 NHFs were investigated by ac/dc conductivity, dielectric constant, dielectric loss and tangent loss as functions of variable frequency, temperature and substitution ratios. Thus, measurements of all dielectric parameters were carried out in the frequency range up to 3.0 MHz and at temperatures between 20 and 120 °C.
3.3 AC conductivity
Both the conduction and mobility charging mechanisms can be elucidated by the ac conductivity study, which contributes to the performance of charge carriers under the influence of a variable electric field. The frequency dependent conductivity of almost all SrGdxFe12−xO19 NHFs based on the substitution ratios up to 0.05 can be evaluated by the following equation [25]:
where \(\varepsilon ^{\prime}\) is the relative permittivity, ε0 is the absolute permittivity, T is the measured temperature, ω is the angular frequency of the externally applied electric field and tanδ is the tangent loss.
The 3D characterization of ac conductivity of SrGdxFe12−xO19 NHFs based on a variety of substitution ratios is shown in Fig. 4. Variations in the substitution ratios of Gd3+ ions, ranging from 0.00 to 0.05, have a great influence in modifying the conduction mechanism as functions of frequencies and temperatures. The general trend in ac conductivity variation shows the known conduction mechanism behaviour given in the above formula for all SrGdxFe12−xO19 NHFs. The substitution of the Gd3+ ion into Sr NHFs were observed to get rid of the fluctuation and to improve the tendencies in conductivity across the temperature variation at higher frequencies. The conductivity increases with the rise in the temperatures at lower frequencies for all Sr NHFs while remaining almost unchanged in higher frequencies. Therefore, Gd3+-ion substitution has a great effect on Sr-ferrite structures that causes that the conduction mechanism. It was observed that the conductivity obeyed the power law as follows [26]:
where σdc (T) is the temperature-dependent dc conductivity and n is the power exponent. The conduction mechanisms for all Sr NHFs have a similar tendency to electric field variations with significant effects of substitution ratios.
3.4 Activation energy and DC conductivity
The 2D and 3D Arrhenius plots of dc conductivity for the SrGdxFe12−xO19 NHFs are depicted in Fig. 5a, b at temperatures up to 120 °C. In the 2D characteristic representation, the dc conductivity is observed to increase with a rise in the substitution ratio and expresses a drop with the reciprocal temperatures. In general, the graphical Arrhenius representation shows that the two regions in activation energy occur along the increase of Gd3+-ion substitution ratios in Sr NHFs. The similar trend is more clearly represented in the 3D Arrhenius plots in Fig. 5b.
Figure 5c clearly shows that the activation energy varies by two phenomenological tendencies with the Gd3+ ion substitution ratio of Sr NHFs as shown in Fig. 5c. Two regions were observed in the activation energy above and below 400 meV according to Gd3+-ion substitution ratio up to 0.05. Linear tendencies in the activation energy were revealed in the low temperature region with values lower than 400 meV while fluctuated trends were measured in the high temperature region resulting in values over 400 meV up to 1459.1 meV. To put it more precisely, as the ratio of Gd3+ ions substituted into Sr NHFs increases, the activation energy on the high temperature side first increases rapidly from 440.8 to 970.1 meV, then decreases slowly to 568.7 meV and finally jumps abruptly to the maximum value of 1459.1 meV. Therefore, the relaxation process in the substituted Sr NHFs could be correlated with various defects in the conduction mechanism of charge carriers because of the Gd substitution ions. Consequently, the dc conductivity for each of the SrGdxFe12−xO19 NHFs can be expressed for the low and high T side as follows:
where σ0(x) is the conductivity at absolute temperature, kB is the Boltzmann constant, x is the Gd3+-ion substitution ratio, Ea(x) is the activation energy in eV and kBT is the thermal energy. It can be clearly seen that the relaxation temperatures associated with the Gd3+ ion substitution ratio affect the activation energy. It should be noted that such a trend is very important in the electrical properties of the entire Sr NHFs. Therefore, the drift mobility of charged carriers also affects from the increase in the Gd3+-ion substitution ratio. As seen clearly in the graph, the temperature increase in Sr NHFs shows the change in the thermal energy of the charge carriers and the change trend in the activation energy. Therefore, a suitable interpretation of this conduction mechanism can only be made by considering the structural effect of both the substituted and the host ion distributions in tetrahedral and octahedral regions. Due to the influence of various Gd3+-ion substitution ratios in the Sr NHFs, the conduction mechanisms can mainly be attributed to the grain-grain boundaries. Thus, the conduction performance in Gd3+-ion-substituted Sr NHFs can be explained significantly by the “Verwey Mechanism” [27].
3.5 Dielectric constant
The 3D characteristic representation of the dependence of the dielectric constant on both frequency and temperature for SrGdxFe12−xO19 NHFs is shown in Fig. 6 for “x” substitution ratios between 0.00 and 0.05. The 3D representation offers a frequency of up to 3.0 MHz between 20 and 120 °C. It is clear that the dielectric constant of all Sr NHFs decreases in different ways with the increasing frequency. However, their decay tendency as a function of frequency is almost different for all Sr NHFs depending on the “x” and the temperature.
For SrGdxFe12−xO19 NHFs (for x = 0.0), there is a sharp drop in temperature of about 60 °C for the central frequencies and then the drop becomes common across both the high frequency and low temperature values. Another sharp drop is observed at higher frequencies. When the measurement frequencies are reached above 1 MHz, it completely varies depending on the overall temperatures up to 120 °C. For the substituted Sr NHFs, a sharp deterioration in the dielectric constant was noticed in the overall frequency range up to MHz levels for the entire Gd3+-ion substitution rates. Although the temperature dependence appears insignificant at higher frequencies, it becomes more effective at lower frequencies. The effect of increasing temperature on the dielectric constant decreases with increasing frequency according to the observed results. It should be also noted that as the frequency increases, the dielectric constant decreases to a certain value, varying by the substitution ratios. At high frequencies, almost all composites show a sharp decline. It should be noted that the dielectric constant generally obeys the power exponent law as follows: \(\varepsilon ^{\prime}\left( {\omega ,T;x} \right)\alpha \omega ^{{n\left( x \right)}}\) where ω is the angular frequency of the externally applied electric field. In addition, it is seen that the dielectric constant increases with increasing Gd3+ ion substitution ratio. So, it can be concluded that the characteristic variation of the dielectric constant in this study can be attributed to the occurrence of electrode interface polarization processes [28].
3.6 Dielectric loss
A 3D representation of the dielectric loss of SrGdxFe12−xO19 NHFs with respect to their ratio of substitution is shown in Fig. 7. 3D characteristic graphs on the subject are represented as a function of temperature up to 120 °C and frequency up to 3.0 MHz. It has been observed that the dielectric loss for Sr NHFs fluctuates throughout the frequency change for the entire temperature range up to 120 °C. For Sr NHFs with a substitution ratio of x = 0.01, the dielectric loss increases with the temperature at low frequencies and remains constant during temperature change at higher frequencies but fluctuates with incremental frequencies. For all other substituted Sr NHFs, the loss increases with the rise of temperature at low frequencies. The temperature dependence of the dielectric loss for all substitution ratios varies in a different way. Initially, it decreases with the frequency and then increases after reaching the minimum value. The structural basis of dielectric ferrite is considered to be composed of very fine conductive ferrite grains electrically insulated with fine-grained boundary layers. Such a variation in dielectric loss in rare earth ion substituted Sr NHFs can be explained using Koop’s theory based on the Maxwell–Wagner model [29, 30].
3.7 Dissipation factor
The dissipation factor, in other words, the dielectric tangent loss (the ratio of the dielectric loss to the dielectric constant) of SrGdxFe12−xO19 (x = 0.00–0.05) NHFs as a function of frequency at different temperatures is illustrated in Fig. 8. From the first plot of the figure (x = 0.00), the logarithmic tangent loss fluctuates considerably with the frequency and temperature, but less fluctuation in temperature change is observed for lower frequencies. For all substituted Sr NHFs, the dissipation factor for the given Gd3+-ion substitution ratios present certain trends depending on both temperature and frequency changes. Furthermore, the tangential loss, with increasing frequency for all temperature values, usually decreases sharply first, then reaches the minimum value and eventually starts to increase again. Especially for x = 0.01, this type of behaviour is slightly varied. Such trends in the tangential loss can be attributed to an effective dipole driven by the alternating electric field. It can be concluded that all trends in dielectric parameters may often differ for variable parameters such as frequency, temperature and substitution ratios. Therefore, by using both the temperature and frequency dependencies of the dissipation factor with the substitutional modification, NHFs can be utilized in technological applications, such as various gas, biosensors and some contrast agents for MRI devices.
4 Conclusion
Gd3+-substituted Sr-hexaferrites were fabricated by citrate sol–gel auto-combustion route. XRD, SEM, TEM and EDX results confirmed the formation of hexagonal crystal structure. The ac conductivity of Sr NHFs is observed to be strongly dependent on the substitution ratio. It can be also stated that conductivity variation generally obeys the power law rule of frequency despite some changes in the substitution ratios. Linear tendencies in activation energy were revealed in the low temperature region with values lower than 400 meV while fluctuated trends were measured in the high temperature region resulting in values over 400 meV up to 1459.1 meV. For all substituted Sr NHFs, the dissipation factor for the given Gd3+ ion substitution ratios present certain trends depending on both temperature and frequency changes. As a result, the trends in the electrical and dielectric parameters examined in this study can offer us many possibilities for certain technological applications such as various gas and biosensors, contrast agents for MRI devices and magnetic hyperthermia.
References
C. Robert, Pullar, hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 57, 1191–1334 (2012)
S. Kumar, M. Kumar Manglam, S. Supriya, H. Kumar Satpal, R. Kumar Singh, M. Kar, Lattice strain mediated dielectric and magnetic properties in La doped barium hexaferrite. J. Magn. Magn. Mater. 473, 312–319 (2019)
M.A. Almessiere, B. Unal, Y. Slimani, A. Demir Korkmaz, N.A. Algarou, A. Baykal, Electrical and dielectric properties of Nb3+ ions substituted Ba-hexaferrites. Res. Phys. 14, 102468 (2019)
M. Amini, A. Gholizadeh, Shape control and associated magnetic and dielectric properties of MFe12O19 (M=Ba, Pb, Sr) hexaferrites. J. Phys. Chem. Solids 147, 109660 (2020)
Y. Slimani, B. Unal, M.A. Almessiere, A. DemirKorkmaz, A. Baykal, Investigation of AC susceptibility, dielectric and electrical properties of Tb–Tm co-substituted M-type Sr hexaferrites. Mat. Chem. Phys. 260, 124162 (2021)
H. Khanduri, M. Chandra Dimri, H. Kooskora, I. Heinmaa, G. Viola, H. Ning, M.J. Reece, J. Krustok, R. Stern, Structural, dielectric, magnetic, and nuclear magnetic resonance studies of multiferroic Y-type hexaferrites. J. Appl. Phys. 112, 073903 (2012)
A. Zafar, S. Shahzada, A. Rahman, S. Anwar, M. Khan, A. Nisar, M. Ahmad, S. Karim, Electrical and magnetic properties of nano-sized Eu doped barium hexaferrites. J. Alloys Compd. 727, 683–690 (2017)
A.V. Trukhanov, V.G. Kostishyn, L.V. Panina, S.H. Jabarov, V.V. Korovushkin, S.V. Trukhanov, E.L. Trukhanov, Magnetic properties and Mössbauer study of gallium doped M-type barium hexaferrites. Ceram. Int. 43, 12822–12827 (2017)
S.V. Trukhanov, A.V. Trukhanov, V.A. Turchenko, An.V. Trukhanov, E.L. Trukhanova, D.I. Tishkevich, V.M. Ivanov, T.I. Zubar, M. Salem, V.G. Kostishyn, L.V. Panina, D.A. Vinnik, S.A. Gudkov, Polarization origin and iron positions in indium doped barium hexaferrites. Ceram. Int. 44, 290–300 (2018)
M.A. Almessiere, Y. Slimani, N.A. Tashkandi, A. Baykal, M.F. Saraç, A.V. Trukhanov, İ Ercan, İ Belenli, The effect of Nb substitution on magnetic properties of BaFe12O19 nanohexaferrites. Ceram. Int. 45, 1691–1697 (2019)
H. Sözeri, Z. Mehmedi, H. Kavas, A. Baykal, Magnetic and microwave properties of BaFe12O19 substituted with magnetic, non-magnetic and dielectric ions. Ceram. Int. 41, 9602–9609 (2015)
A. Baykal, İS. Ünver, U. Topal, H. Sözeri, Pb substituted Ba Sr-hexaferrite nanoparticles as high quality microwave absorbers. Ceram. Int. 43(16), 14023–14030 (2017)
S.V. Trukhanov, A.V. Trukhanov, M.M. Salem, E.L. Trukhanova, L.V. Panina, V.G. Kostishyn, M.A. Darwish, T.I. Zubar, D.I. Tishkevich, V. Sivakov, D.A. Vinnik, S.A. Gudkova, C. Singh, Preparation and investigation of structure, magnetic and dielectric properties of (BaFe11.9Al0.1O19)1–x - (BaTiO3)x bicomponent ceramics. Ceram. Int. 44, 21295–21302 (2018)
M.A. Darwish, A.I. Afifi, A.S. Abd El-Hameed, H.F. Abosheiasha, A.M.A. Henaish, D. Salogub, A.T. Morchenko, V.G. Kostishyn, V.A. Turchenko, A.V. Trukhanov, Can hexaferrite composites be used as a new artificial material for antenna applications? Ceram. Int. 47, 2615–2623 (2021)
S. Nemrava, D.A. Vinnik, Z. Hu, M. Valldor, C.-Y. Kuo, D.A. Zherebtsov, S.A. Gudkova, C.-T. Chen, L.H. Tjeng, R. Niewa, Three oxidation states of manganese in the barium hexaferrite BaFe12–xMnxO19. Inorg. Chem. 56, 3861–3866 (2017)
M. Häßner, D.A. Vinnik, R. Niewa, Structure and magnetic properties of a new hexaferrite (Ba, Pb)(Fe, Ti)9O15. Ceram. Int. 47, 5341–5346 (2021)
Y. Slimani, N.A. Algarou, M.A. Almessiere, A. Sadaqat, M.G. Vakhitov, D.S. Klygach, D.I. Tishkevich, A.V. Trukhanov, S. Güner, A.S. Hakeem, I.A. Auwal, A. Baykal, A. Manikandan, I. Ercan, Fabrication of exchanged coupled hard/soft magnetic nanocomposites: correlation between composition, magnetic, optical and microwave properties. Arab. J. Chem. 14(3), 102992 (2021)
N.A. Algarou, Y. Slimani, M.A. Almessiere, S. Güner, A. Baykal, I. Ercan, P. Kögerler, Exchange-coupling effect in hard/soft SrTb0.01Tm0.01Fe11.98O19/AFe2O4 (where A=Co, Ni, Zn, Cu and Mn) composites. Ceram. Int. 46, 7089–7098 (2020)
N.A. Algarou, Y. Slimani, F.S. Alahmari, M.G. Vakhitov, D.S. Klygach, S.V. Trukhanov, A.V. Trukhanov, A. Baykal, Magnetic and microwave properties of SrFe12O19/MCe0.04Fe1.96O4 (M = Cu, Ni, Mn, Co and Zn) hard/soft nanocomposites. J. Mater. Res. Technol. 9(3), 5858–5870 (2020)
H. Khanduri, M. Chandra Dimri, H. Kooskora, I. Heinmaa, G. Viola, H. Ning, M.J. Reece, J. Krustok, R. Stern, Structural, dielectric, magnetic, and nuclear magnetic resonance studies of multiferroic Y-type hexaferrites. J. Appl. Phys. 112, 073903 (2012)
A.V. Trukhanov, V.G. Kostishyn, L.V. Panina, V.V. Korovushkin, V.A. Turchenko, P. Thakur et al., Control of electromagnetic properties in substituted M-type hexagonal ferrites. J. Alloys Compd. 754, 247–256 (2018)
N. Rezlescu, C. Doroftei, E. Rezlescu, P.D. Popa, The influence of heat-treatment on microstructure and magnetic properties of rare-earth substituted SrFe12O19. J. Alloys Compd. 451, 492–496 (2008)
N. Rezlescu, E. Rezlescu, C. Pasnicu, M.L. Craus, Improvement of the magnetic properties for Mn–Ni–Zn ferrites by rare earth Nd3+ ion substitution. J. Phys. 6, 5707–5716 (1994)
L.F. Chen, C.K. Ong, C.P. Neo, V.V. Varadan, V.K. Varadan, Microwave Electronics: Measurement and Materials Characterization (Wiley, Hoboken, 2004)
B. Unal, M.A. Almessiere, Y. Slimani, A. Baykal, A.V. Trukhanov, I. Ercan, The conductivity and dielectric properties of neobium substituted Sr–NHFs. Nanomaterials 9, 1168 (2019)
N.A. Algarou, Y. Slimani, M.A. Almessiere, S. Rehman, M. Younas, B. Unal, A. Demir Korkmaz, M.A. Gondal, A.V. Trukhanov, A. Baykal, I. Nahvi, Developing the magnetic, dielectric and anticandidal characteristics of SrFe12O19/x(Mg0.5Cd0.5Dy0.03Fe1.97O4) hard/soft ferrite nanocomposites. J. Taiwan Inst. Chem. Eng. 113, 344–362 (2020)
P. Piekarz, K. Parlinski, A.J.M. Oleś, Mechanism of the verwey transition in magnetite. Phys. Rev. Lett. 97, 156402 (2006)
Y. Slimani, B. Unal, M.A. Almessiere, A. DemirKorkmaz, A. Baykal, Investigation of AC susceptibility, dielectric, and electrical properties of Tb-Tm co-substituted M-type Sr hexaferrites. Mater. Chem. Phys. 260, 124162 (2021)
T. Honegger, K. Berton, E. Picard, D. Peyrade, Determination of Clausius–Mossotti factors and surface capacitances for colloidal particles. Appl. Phys. Lett. 98, 181906 (2011)
C.G. Koops, On the dispersion of resistivity and dielectric constant of some semiconductors at audio frequencies. Phys. Rev. 83, 121–124 (1951)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ünal, B., Almessiere, M.A., Slimani, Y. et al. A study on the electrical and dielectric properties of SrGdxFe12−xO19 (x = 0.00–0.05) nanosized M-type hexagonal ferrites. J Mater Sci: Mater Electron 32, 18317–18329 (2021). https://doi.org/10.1007/s10854-021-06373-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06373-9