Abstract
V/Ni co-doped zinc ferrite (Zn1-yNiyFe2-xVxO4, x = 0.01, 002, 0.03, 0.04, 0.05; y = 0.05, 015, 0.25) ceramics were prepared by solid-state reaction method. The effect of V and Ni contents on the electrical property and aging property of the ceramics were investigated. All the ceramics have a cubic spinel structure and show typical characteristics of negative temperature coefficient (NTC) of resistivity. For being doped with various V and Ni contents, the room temperature resistivities (ρ25) of the ceramics range from 0.037 MΩ·cm to 4.8 MΩ·cm, and NTC material constants (B values) vary between 3255 K and 6614 K. The Zn1-yNiyFe2-xVxO4 ceramic with x = 0.04 and y = 0.15 has high electrical stability with a resistance drift rate of 0.19% after having been aged at 150 °C for 500 h in air. The aging characteristic was investigated by analyzing cationic valences using X-ray photoelectron spectroscopy before and after aging treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Negative temperature coefficient (NTC) thermistors have characteristics of decreasing resistivity with temperature increasing. For their unique properties, NTC thermistors are widely applied in fields such as temperature monitoring, temperature compensation, surge current suppression, bolometric detection, and infrared imaging [1,2,3]. In recent years, the investigations of NTC thermistors are mostly based on AB2O4 spinel manganates and perovskite compounds [4,5,6,7]. NTC ceramics based on simple oxide-based semiconductors have also attracted attention due to their unique characteristics [8,9,10]. Material constant (B value) representing temperature sensitivity, room temperature resistivity (ρ25) and electrical stability are important parameters for NTC thermistors. Different B values and ρ25 are required for various applications. It is reported that the properties of NTC thermistors can be adjusted and improved by cation doping or process improvement [11,12,13,14,15,16,17]. For examples, Xiong et al. reported that the Mn1.5−0.5xCo0.9−0.3xNi0.6−0.2xCuxO4 ceramics prepared by heterogeneous precipitation have adjustable B values and ρ25 by substitution with different contents of Cu ions [15]. Wang et al. reported that the B value and ρ25 of Co2.77Mn1.71Fe1.10Zn0.42O8 ceramics could be adjusted by changing sintering temperatures [17].
In the meanwhile, electrical stability is also a key property for NTC thermistors. Many studies have reported on the aging phenomena of NTC ceramics. Grone et al. proposed two aging models basing on the study on the Mn–Ni–Fe–O system [18, 19], i.e., absorption or release of oxygen, and redistribution of cations at the sublattices. The effect of chemical composition on the aging of NixMn3−xO4+δ (0.56 ≤ x ≤ 1.0) ceramics was studied by Fang et al. [20], the ceramics with a nickel content of less than 0.7 showed high electrical stability with resistance drift rates less than 0.5%. The composition dependence of aging performance is proved by using the conductive mechanism of small polaron theory and the thermodynamic optimal position of cation redistribution [20]. Recently, Zhao et al. studied the influence of Cu ions on aging property of Ni0.7Mn2.3-xCuxO2 (x = 0.1, 0.2, 0.3, and 0.4) NTC ceramics by analyzing the change of cationic valences using X-ray photoelectron spectroscopy (XPS) [21]. Their results showed that Cu-ionic dopant greatly reduced the resistivity and B value, but, the rise of Cu-ionic content increased the adding-induced drift in both resistivity and B value, resulting from the change of Cu-valence from Cu+ to Cu2+ as well as the decrease of Mn4+ content after aging.
Spinel-type ferrites (denoted as MFe2O4, M = Ni, Co, Mg, Cu, Mn, Zn, etc.) such as CuFe2O4 and MnFe2O4-based ceramics also have good NTC properties [22, 23]. The CuFe2O4-based ceramics reported by Liu et al. showed that the B value can be adjusted from 2971 K to 4180 K by altering the content of the Mo ions additive [22]. As reported by Guo et al. [23], the properties of MnFe2O4-based ceramics can also be modified by using Mo ions additive and the B value reached about 6100 K. The conduction mechanism of those compounds was proposed to follow the polaron hopping model, namely Fe3+ + Fe2+ ↔ Fe2+ + Fe3+ and Mn3+ + Mn2+ ↔ Mn2+ + Mn3+. However, to our knowledge, there has no report on the aging stability of the NTC thermistors based on spinel-type ferrites so far. In this work, V/Ni co-doped ZnFe2O4 ceramics were synthesized, the electrical properties and aging characteristics were investigated. The results show that ZnFe2O4 ceramics have a typical NTC effect, and room temperature resistivity (ρ25) and temperature sensitivity constant (B value) can be adjusted in a certain range. The co-doped samples show better aging stability, the composition and valence of elements were analyzed by XPS, and the aging effect of ZnFe2O4-based ceramics were discussed.
2 Experimental
2.1 Sample preparation
The nominal composition of ZnFe2-xVxO4 (x = 0, 0.01, 0.02, 0.03, 0.04 and 0.05; denoted as A0, A1, A2, A3, A4 and A5, respectively) and Zn1-yNiyFe1.96V0.04O4 (y = 0.05, 0.15 and 0.25; denoted as S1A4, S2A4 and S3A4, respectively) ceramics were prepared by conventional solid-state reaction method. Zinc oxide (ZnO, ≥ 99.0%, Chinese pharmaceutical chemical reagent, China), iron citrate (FeC6H5O7·5H2O, ≥ 97%, Chinese pharmaceutical chemical reagent, China), vanadium pentoxide (V2O5, > 99%, Sinopharm Chemical Reagent Co, Ltd, China) and basic nickel carbonate (NiCO3·2Ni(OH)2·4H2O, ≥ 99.0%, Xilong Scientific, China) were used as the raw materials. For each batch of the experiment, stoichiometric raw materials were weighed according to the designed composition. The starting materials were mixed with ball-milling, and the slurry was dried at 110 °C for 24 h. Then, mixtures were calcined in air at 900 °C for 5 h. The calcined powders were granulated with polyvinyl alcohol solution and pressed into disks with a diameter of 12 mm and thickness of about 2 mm. The green pellets were sintered in air at 1350 °C for 1 h. To test the electrical properties, two opposite surfaces of the as-sintered ceramics were polished and then coated with silver paste followed by being heated at 600 °C for 5 min to make the ohmic electrodes.
2.2 Characterization
Phase components of the sintered ceramics were detected by X-ray diffraction (XRD, Rigaku D/max 2500, Japan) at room temperature. The diffraction angle 2θ was selected in the range between 10° and 80°. The densities of sintered samples were measured by the Archimedes drainage method. The microstructure of ceramics was observed by using a field emission scanning electron microscopy (FESEM, sirion200). In SEM observation, the sintered ceramics were broken into pieces and the fracture morphology was observed. X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher, USA) was used to analyze possible valences of cations in ceramics. The standard C 1 s peak (binding energy of 248.8 eV) was used for calibration.
Temperature dependence of resistance of all ceramics was measured at 25 °C–250 °C using a resistance temperature measurement system (ZWX-C, China) under direct current (DC) condition. The resistivity (ρ) was calculated based on the measured resistance (R) according to the equation of ρ = R(S/h), where S and h are, respectively, electrode area (cm2) and thickness (cm) of the measured sample. An electrochemical test system (Gamry reference 600, USA) was used to measure complex impedance spectra of ceramics. Impedance data were analyzed by Gamry Echem Analyst program. To test electrical stability, the aging treatment of samples with ohmic electrodes was performed at 150 °C for 500 h in air. The resistances were measure at room temperature (25 °C). The resistance drift rate was calculated by [(RA–R0)/R0] × 100%, where R0 is original resistance and RA is resistance after being aged.
3 Results and discussion
3.1 Phase and microstructure
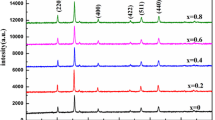
Figure 1 shows XRD patterns of some typical ceramics. All XRD patterns show cubic spinel phase with space group Fd \(\overline{3 }\) m (227) (Ref. PDF No. 79–1150). In the partially enlarged illustration shown in the inset, slight impurity peaks reflected from the NiV2O6 phase (Ref. PDF No. 76–0359) can be detected only in the S3A4 sample. Refined by the Jade-6 analysis program, the related lattice parameters are obtained and shown in Table 1. The lattice parameters changed due to doping with V or Ni ions, indicating that V and Ni ions have substituted into the spinel lattice. The lattice parameters of ZnFe2O4 ceramics decrease with the increase of V-ionic content, mainly because the radius of V5+ ion (0.059 nm) [24] is smaller than that of Fe3+ ion (0.064 nm) [22] and Zn2+ ion (0.074 nm) [27]. With the increase of Ni ionic content, the lattice parameter continues to decrease. When y is 0.25 in Zn1-yNiyFe1.96V0.04O4, impurity NiV2O6 was detected, indicating the content of Ni for y = 0.25 exceeds the related solid solubility in the studied ceramic.
SEM images of samples A0, A4, S2A4 and S3A4 are shown in Fig. 2. It can be seen that the grains are closely connected to each other, evenly distributed, and few pores can be found. According to measurement by the Archimedes method, relative densities of ceramics were obtained and are shown in Table 1. The relative density of doped samples is higher than that of undoped sample, which should be attributed to the existence of V2O5 which has low melting point and promotes the sintering.
3.2 Electrical properties
Figure 3a shows the temperature dependence of resistivity of ZnFe2-xVxO4 with different V contents. All ceramics show typical NTC characteristics. Each plot is approximately linear and follows the Arrhenius relation as shown in Eq. (1).
where ρ is resistivity at temperature T (in Kelvin), ρ0 is a factor related to the material characteristics, k is the Boltzmann constant, Ea is the activation energy of conduction, and B is a material constant representing the temperature sensitivity. B value can be calculated by Eq. (2).
where ρ1 and ρ2 are resistivities at T1 and T2, respectively. T1 and T2 are often chosen as 298 K (25 °C) and 358 K (85 °C). So the B value is usually written as B25/85.
Figure 3b shows ρ25 and B as a function of V content. The detailed data are listed in Table 2. All B are more than 3000 K. Doping of V5+ ions significantly reduced ρ25. This should result from the doping effect of semiconductor for that the substitution of V5+ ions introduced electrons. The related defect reaction can be described as Eq. (3).
The introduced electrons act as weakly bound electrons and locate at the donor level, and can be thermally activated to jump to the conduction band, resulting in increase of conductivity. In the meanwhile, doping with V ions, B values increased. For the ionic substitution, the different radii between V and Fe ions resulted in lattice distortion and increase of lattice potential. The carrier migration barrier is increased, resulting in higher Ea and B.
Figure 4a shows the resistivity as a function of temperature for V/Ni co-doped ZnFe2O4 (Zn1-yNiyFe1.96V0.04O4) ceramics with different Ni content. The ceramics show typical NTC characteristics. The resistivity increases with the increase of Ni content. When the Ni contents (y) are 0, 0.05, 0.15 and 0.25, ρ25 are 37.1, 744.6, 3050.3 and 4800.4 kΩ·cm, respectively. Figure 4b and Table 2 show the ρ25 and B dependence of Ni-ion concentration in Zn1-yNiyFe1.96V0.04O4 ceramics. It can be seen that Ni substitution increased ρ25 and B. All B values are higher than 3000 K and are suitable for practical applications (2000 – 5000 K) [1].
Figure 5 shows the complex impedance spectra in Cole–Cole plots of A0, A4 and S2A4 ceramics measured at room temperature. Where, the impedances of both real part and imaginary part were calculated taking account into the sample size, so the unit of impedance is kΩ·cm. An equivalent circuit shown in the inset is used to fit the impedance data. Among them, Rg and Rgb represent the impedance of grain boundary effect and grain boundary effect, respectively. CPE1 and CPE2 represent the constant phase elements of grain effect and grain boundary effect, respectively, and are explained as the deviation of capacitance from ideal behavior. Fitted with the equivalent circuit, the resistivities of grain effect and grain boundary effect of each sample are shown in Table 3. Doping of V ion reduced the resistivities of both grain effect and grain boundary effect in the ceramics, while, Ni-doping increased the resistance of both grain effect and grain boundary effect. These also indicate that V and Ni ions have substituted into the lattice, although impurity phase of NiV2O6 in S3A4 ceramic were detected as shown in Fig. 1. The impurities might locate at grain boundary and result in the resistance increase.
To determine the effect of cationic valences on the electrical properties of the ceramics, XPS spectra were analyzed as shown in Fig. 6. The full XPS spectra are shown in Fig. 6a. The characteristic peaks of Zn, Fe, O and Ni, V can be observed. The XPS spectra were analyzed in detail by Gaussian Lorentz curve fitting. Figure 6b shows V 2p orbital energy peaks of A4 and S2A4 samples. The XPS peak of V 2p3/2 is composed of two splitting peaks (516.27 eV and 517.21 eV), which correspond to V4+ and V5+ ions [25]. The ratios of XPS peaks of V4+/V5+ are 1.34:1 and 1.32:1 in A4 and S2A4 ceramics, respectively. This indicates that the introduction of Ni ions has almost no effect on the concentrations of V4+ and V5+ ions.
The Fe 2p spectra of A0, A4 and S2A4 samples are shown in Fig. 6c. Each 2p3/2 orbital can be fitted by two characteristic peaks, indicating that there are two valence states of Fe ion (Fe2+ and Fe3+) in each sample. The splitting peak positions are at 710.15 eV and 711.46 eV, corresponding to Fe2+ and Fe3+, respectively [26]. The ratios of XPS peaks of Fe2+/Fe3+ in A0, A4 and S2A4 ceramics are 0.54:1, 0.95:1 and 0.69:1, respectively. The existence of high content of Fe2+ ions in the A0 (ZnFe2O4) ceramic might originate from the processes such as calcining and sintering under an insufficient oxygen atmosphere, leading to the formation of oxygen vacancies. The defect reaction can be described as Eq. (4).
So the formula of ZnFe2O4 could be expressed as \({\text{ZnFe}}_{\varepsilon }^{2+}{\text{Fe}}_{2-\varepsilon }^{3+}{\text{O}}_{4-\frac{\varepsilon }{2}}\), where ε is the quantity of oxygen vacancies. The occurrence of Fe2+ ions enhanced the conductivity for the polaron hopping model [22, 23]. These should be the main reason for the conduction of undoped ZnFe2O4 ceramic as shown in Fig. 3. The introduction of V ions (V4+ and V5+) induced the increase of Fe2+ ion concentration for compensating electric neutrality of ceramic. The increase of Fe2+/Fe3+ ionic ratio induced by V ions provided high polaron hopping chance between Fe2+ and Fe3+ ions, combining with V-induced production of weakly bound electrons as discussed in Eq. (3). So the conductivity was enhanced by doping with V ions as shown in Fig. 3.
Figure 6d shows the Ni 2p narrow XPS spectrum in S2A4 ceramic. The spectrum consists of a satellite peak and Ni 2p3/2 peak. The Ni 2p3/2 peak was fitted to be composed of two splitting peaks with binding energies of 854.50 eV and 856.20 eV, which correspond to Ni2+ and Ni3+ valence states, respectively [27]. The transition from Ni2+ to Ni3+ ions should be accompanied by the formation of holes. At the same time, for compensating electric neutrality, the content of Fe2+ ions was reduced comparing with A4 ceramic.
3.3 Aging properties
The resistance drift rates (ΔR/R0) of Zn1-yNiyFe2-xVxO4 ceramics (x = 0.01, 002, 0.03, 0.04, 0.05; y = 0.05, 015, 0.25) after being aged at 150 °C for 500 h are shown in Fig. 7. In the initial stage, the resistance increased with the increase of aging time and tended to be stable after 200 h. The resistance drift rate of the A4 sample reached about 6.20% and the one A0 sample is 4.68% after 500 h aging treatment, while the resistance drift rate of S2A4 sample is the smallest one, about 0.19%. It is obvious that Ni-doping may improve obviously electrical stability.
XPS was used to analyze the ionic valence states of the aged ceramics. Figure 8a shows XPS comparison analysis of V ions in A4 and S2A4 samples after aging. Through fitting and calculation, the V4+/V5+ peak ratios of A4 and S2A4 ceramics are 1.43:1 and 1.36:1, respectively. Compared to the results of the samples before aging as shown in Fig. 6b, the concentration of V5+ ions decreased after aging treatment. The change quantity of V5+ concentration in S2A4 ceramic is smaller than that in A4 ceramic.
The valence states of Ni ions of S2A4 before and after 500 h aging were analyzed as shown in Fig. 8b. Each Ni 2p3/2 peak is composed of two splitting peaks with the binding energies of 854.50 eV and 856.20 eV, respectively. These indicate that Ni2+ and Ni3+ ions co-exist in V/Ni-doped ZnFe2O4-based ceramic. The content ratios of Ni2+/Ni3+ were calculated to be 5.96:1 and 4.87:1 before and after aging, respectively. After aging, the quantity of Ni2+ ions decreased and Ni3+ ions increased.
The valence states of Fe ions in A4 and S2A4 samples before and after aging were analyzed as shown in Fig. 8c and d. The concentration ratios of Fe2+ to Fe3+ in A4 and S2A4 samples were calculated, respectively, to be 0.95:1 and 0.69:1 before aging, while the ratios changed to be 0.82:1 and 0.61:1 after aging. These indicate that Ni-doping in the ZnFe2O4-based ceramics enhanced the valent stability of Fe ions.
According to the XPS analysis, the introduction of Ni-ion contributed to the stability of cationic valence. This might result from the electrovalence compensating and rearrangement of ion occupying. Fe, V and Ni are transition metals, and their valent states may change when they are exposed to oxidation or reduction atmosphere, or surface adsorption of gas. As reported by Gao et al. [28], cationic oxidation should be one of the main contributions for resistance drift during the aging process. According to the reports by Bârsan et al. and Dai et al. [29, 30], oxygen molecules (O2) in atmospheric environment adhere to crystal surfaces, then the O2 capture electrons from ceramic surface or interfaces (also be grain boundaries) to turn into the adsorbed oxygen (Oadn−, n ≤ 2). When the adsorbed oxygen diffused into the ceramic body, cations may be oxidized. As the results, cations with higher valence produced, e.g., Ni2+ was oxidized to Ni3+ and Fe2+ was oxidized to Fe3+. For electrovalence compensating, the concentration of V5+ decreased as shown in Fig. 8a. On the other hand, the ZnFe2O4 itself has an inverse spinel structure, Ni2+, Ni3+, Fe2+ and Fe3+ ions may occupy at either oxygen octahedral interstices or oxygen tetrahedral interstices. So cation redistribution might take place during aging, leading to the conductivity change and resistance drift. As analyzed in Fig. 8c and d, the introduction of Ni-ion restrained valence evolution of Fe ions, reduced the extent of cation redistribution, resulting in higher electrical stability during aging.
4 Conclusion
The V/Ni co-modified ZnFe2O4 ceramics (ZnFe2-xVxO4, x = 0, 0.01, 0.02, 0.03, 0.04 and 0.05; Zn1-yNiyFe1.96V0.04O4, y = 0.05, 0.15 and 0.25) with spinel structure were prepared by solid-state reaction method. By changing the contents of Ni and V ions, the room temperature resistivity ranging between 0.037 MΩ·cm and 4.8 MΩ·cm and NTC material constant of B ranging between 3255 K and 6614 K were obtained. V/Ni co-modifying contributes to higher electrical stability of ZnFe2O4-based ceramics during aging. The Zn0.85Ni0.15Fe1.96V0.04O4 ceramic showed the highest electrical stability with a resistance drift rate of 0.19% after being aged at 150 °C for 500 h in air, while the ZnFe2O4 and ZnFe1.96V0.04O4 sample have 4.68% and 6.20% of resistance drift rates. The introduction of Ni-ion reduced the oxidation extent of Fe ions and is proposed to restrain the extent of cation redistribution, resulting in higher electrical stability during aging. It shows that Zn1-yNiyFe2-xVxO4 spinel system has great potential as a candidate for applications for NTC thermistors.
References
A. Feteira, Negative temperature coefficient resistance (NTCR) ceramic thermistors: an industrial perspective. J. Am. Ceram. Soc. 92 (2009) 967–983.
M. Schubert, C. Münch, S. Schuurman, V. Poulain, J. Kita, R. Moos, Characterization of nickel manganite NTC thermistor films prepared by aerosol deposition at room temperature. J. Eur. Ceram. Soc. 38 (2018) 613–619.
H. Gao, C. Ma, B. Sun, Preparation and characterization of NiMn2O4 negative temperature coefficient ceramics by solid-state coordination reaction. J. Mater. Sci. Mater. Electron. 25 (2014) 3990–3995.
H.S. Han, K.R. Park, Y.R. Hong, K. Shim, S. Mhin, Effect of Fe incorporation on cation distributions and hopping conductions in Ni-Mn-Co-O spinel oxides. J. Alloys Compd. 732 (2018) 486–490.
C. Ma, H. Gao, TEM and electrical properties characterizations of Co0.98Mn2.02O4 NTC ceramic, J. Alloys. Compd. 749 (2018) 853–858.
B.Y. Price, G. Hardal, Preparation and characterization of Ni-Co-Zn-Mn-O negative temperature coefficient thermistors with B2O3addition. J. Mater. Sci. Mater. Electron. 30 (2019) 17432–17439.
Y. Zeng, Z. C. Li, J. M. Shao, X. C. Wang, W. B. Hao, H. Zhang, Electrical properties of perovskite YFeO3 based ceramics modified by Cu/Nb ions as negative temperature coefficient thermistors, J. Mater. Sci. Mater. Electron. 30 (2019) 14528–14537.
Z. Yang, H. Zhang, Z. He, B. Li, Z. Li, Influence of B3+- and Na+-ions on electrical property and temperature sensitivity of NiO-based ceramics, J. Mater. Sci. Mater. Electron. 30 (2019) 3088–3097.
B. Yang, H. Zhang, J. Guo, Y. Liu, Z. Li, Electrical properties and thermal sensitivity of Ti-Y modified CuO-based ceramic thermistors. J. Mater. Sci. Mater. Electron. 10 (2016) 413–421.
G. Wang, H. Zhang, X. Sun, Y. Liu, Z. Li, Characterization of a new system of NTC temperature-sensitive ceramics based on Al/F modified NiO simple oxides. J. Mater. Sci. Mater. Electron. 28 (2017) 363–370.
S. Liang, C. Cao, Y. Yuan, H. Li, M. Luo, M. Gao, X. Zhang, Hydrothermal synthesis of Zn-doped Ni-Mn-Al-O thin films toward high-performance negative temperature coefficient thermistor. J. Mater. Sci. Mater. Electron. 29 (2018) 9025–9032.
C. Zhao, Y. Zhao, The investigation of Zn content on the structure and electrical properties of ZnxCu0.2Ni0.66Mn2.14-xO4 negative temperature coefficient ceramics, J. Mater. Sci. Mater. Electron. 23 (2012) 1788–1792.
A.N. Kamlo, J. Bernard, C. Lelièvre, D. Houivet, Synthesis and NTC properties of YCr1−xMnxO3 ceramics sintered under nitrogen atmosphere. J. Eur. Ceram. Soc. 31 (2011) 1457–1463.
S. Liang, D. Zhao, M. Cui, M. Luo, H. Li, X. Zhang, Two-step sintering of submicro-grain Ni0.54Mn1.26Fe1.2O4 NTC ceramics with an excellent electrical performance, J. Mater. Sci. Mater. Electron. 30 (2019) 20144–20153.
K. Xiong, S.X. Zhao, D.F. Li, H.Q. Bao, C.W. Nan, Structure and electrical performance of Mn1.5–0.5xCo0.9–0.3xNi0.6–0.2xCuxO4 NTC ceramics prepared by heterogeneous precipitation, J. Alloys Compd. 606 (2014) 273–277.
W. Fu, Z. Li, P. Li, Y. Zeng, H. Zhang, Electrical property and temperature sensitivity of NiFe2−xSbxO4 (x ≤ 0.02) ceramics for negative temperature coefficient thermistors, J. Mater. Sci. Mater. Electron. 29 (2018) 11637–11645.
B. Wang, J.H. Wang, A.M. Chang, J.C. Yao, Sintering temperature and XPS analysis of Co2.77Mn1.71Fe1.10Zn0.42O8 NTC ceramics, Mater. Chem. Phys. 239 (2020) 122098–122102.
W.A. Grone, V. Zaspalis, S. Schuurman, Aging of NTC ceramics investigated by magnetic measurement. J. Mater. Sci. Lett. 18 (1999) 1233–1235.
W.A. Grone, C. Metzmacher, P. Huppertz, S. Schuurman, Aging of NTC ceramics in the system Mn-Ni-Fe-O. J. Electroceram. 7 (2001) 77–87.
D. Fang, C. Zheng, C. Chen, A.J.A. Winnubst, Aging of nickel manganite NTC ceramics. J. Electroceram. 22 (2009) 421–427.
M. Zhao, W. Chen, W. Wu, M.L. Zhang, Z. Li, Aging characteristic of Cu-doped nickel manganite NTC ceramics. J. Mater. Sci. Mater. Electron. 31 (2020) 11784–11790.
Y. Liu, S.L. Leng, S.H. Li, W.Y. Fu, Z.C. Li, H. Zhang, Electrical properties and temperature sensitivity of Mo-modified CuFe2O4 ceramics, Mater. Res. Express. 5 (2018) 036307.
J. Guo, H. Zhang, Z.L. He, S.H. Li, Z.C. Li, Electrical properties and temperature sensitivity of Mo-modified MnFe2O4 ceramics for application of NTC thermistors, J. Mater. Sci. Mater. Electron. 29 (2018) 2491–2499.
Y. Liu, H. Zhang, W. Fu, Z. Yang, Z.C. Li, Characterization of temperature sensitivity of V-modified CuFe2O4 ceramics for NTC thermistors. J. Mater. Sci. Mater. Electron. 29 (2018) 18797–18806.
G. Silversmit, D. Depla, H. Poelman, G.B. Marin, R.D. Gryse, Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. 135 (2004) 167–175.
T. Yamashita, P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 254 (2008) 2441–2449.
X. Sun, Z. Li, W. Fu, S. Chen, H. Zhang, Li/Fe modified Zn0.3Ni0.7O NTC thermistors with adjustable resistivities and temperature sensitivity, J. Mater. Sci. Mater. Electron. 29 (2018) 343–350.
C. Gao, Z. Li, L. Yang, D. Peng, H. Zhang, Investigation of electrical and aging properties of Bi-modified (Zn0.4Ni0.6)1-xNaxO ceramic thermistors, J. Eur. Ceram. Soc., 41 (2021) 4160–4166.
W. Dai, X. Pan, C. Chen, S. Chen, W. Chen, H. Zhang, Z. Ye, Enhanced UV detection performance using a Cu-doped ZnO nanorod array film. RSC Adv. 4 (2014) 31969–31972.
N. Bârsan, C. Simion, T. Heine, S. Pokhrel, U. Weimar, Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 25 (2010) 11–19.
Acknowledgements
This work is supported by the research funding from the Hunan Wedid Materials Technology Co., Ltd., China (No. 738010241), the National Natural Science Foundation of China (No. 51767021), and the Research and Development program in key areas of Guangdong Province, China (No. 2019B090913002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hao, W., Zhang, H., Gao, C. et al. Influence of V/Ni-doping on electrical properties and aging stability of ZnFe2O4-based NTC ceramics. J Mater Sci: Mater Electron 32, 17800–17809 (2021). https://doi.org/10.1007/s10854-021-06316-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06316-4