Abstract
The fabrication of functional nanocomposite films by electrically driven techniques is an emerging method that has attracted increasing research interest because of advantages such as easy operation, low-cost, no secondary pollution. In this study, electrophoretic assembly technique (EAT) was firstly developed to design the promising novel nano-aluminum@tungsten trioxide (nano-Al@WO3) energetic coating with even distribution of nanoparticles under room temperature and pressure conditions, using the mixed liquor of isopropyl alcohol, PEG-1000, and polyethyleneimine as the optimal dispersion liquid for EAT. High crystallinity and purity of product was demonstrated by X-ray diffractometer. Differential scanning calorimetry (DSC) results that the obtained Al@WO3 was an outstanding high-energy coating with a high output of heat (~ 2400 J/g) due to the sufficient thermite reaction between nano-Al and WO3 particles, and the heat-release process of product can be highly controlled by adjusting the solid loading concentration ratio of nano-Al@WO3 before EAT. Thus, the fabrication method of EAT proposed here has great potentials to high-efficient construction or design of various energetic nanocomposite coatings such as thermite and bimetallic reactive composites with wide applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nano-metastable intermixed composites (MICs) or energetic materials are types of reactive nanocomposites that release energy aggressively under certain external energy stimulus due to typical redox reaction between fuels (Al or Mg) and oxidizing agents (such as CuO, Fe2O3, Bi2O3, potassium perchlorate) [1,2,3,4]. Such energetic materials with superior heat-release performance is widely used in blasting, micro propellant, sensor, welding, etc. [5,6,7]. Among them, nano-aluminum@tungsten trioxide (nano-Al@WO3) as a promising MIC has attracted increasing interests because of both extremely fast combustion rate (hundreds of meters per second) and a high theoretical heat (\(\varDelta \text{Q}\)) of reaction (as shown in Eq. 1) [8, 9]:
Because of high-energy release, fabricating nano-Al@WO3 MIC conveniently has become the hot off the press in recent decades. For example, Son et al. [10] designed four kinds of nano-level Al@RxOy (R = W, Mo, Cu, Bi) using ultrasonic mixing method and studied the influence law of their reaction ratio on combustion rate and detonation pressure, respectively. In addition, reactive ball milling technique has been applied to design nano-Al@WO3 MIC, and the influence law of different active carbon-based additives on its deflagration performance was deeply studied by A. Bach group [11]. For practical purpose, design of MIC coating is the key to realize the efficient combination with various substrates or chips. However, how to fabricate nano-Al@WO3 MIC is still an immense challenge via a convenient and efficient technique.
Due to several advantages of convenient operation, low-cost, high film-forming rate, and outstanding feasibility for complex basal shapes, electrophoretic assembly technique (EAT) has exhibited great potential in constructing coatings or films through movement of charged micro/nanoparticles or micromolecules under a suitable electric field [12,13,14,15], which is also successfully proved to be practicable for preparing several MIC coatings (Al@Bi2O3, Al@CuO, Al@Ni, etc. [16,17,18]). Nevertheless, there are no reports on the fabrication of nano-aluminum@tungsten trioxide MIC by EAT.
This is the first study that has designed the nano-Al@WO3 energetic coating through a EAT process. The optimized dispersion system contains the mixture of isopropyl alcohol, PEG-1000, and polyethyleneimine. The micromorphology and crystal structure of target coating were also studied in detail. The thermodynamic properties of nano-Al@WO3 energetic coating were analyzed by differential scanning calorimetry (DSC). Simultaneously, the optimization design of heat-release properties were investigated.
2 Experimental section
2.1 Reagents and materials
Polyethyleneimine, polyvinylpyrrolidone (PVP-40,000), ethanol, and nano-Al particles (99.9%) were purchased from Aladdin Inc. Corp. (Shanghai, China). Isopropyl alcohol was used from Kelong Industrial Inc. (Chengdu, China). The other reagents (including PEG-2000) from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) were of analytical grade purity without further purification. Commercially available nickel sheets are used as electrode materials during EAT process.
2.2 EAT design of nano-Al@WO3 energetic coatings
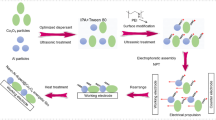
For fabricating the nano-Al@WO3 energetic coating, the EAT technique was exploited, and the mechanism diagram is illustrated in Fig. 1. Firstly, WO3 nanoparticles with the diameter of ~ 50 nm were synthetized by a typical solution using PVP-40,000, PEG-2000, and ammonium metatungstate as precursor reagents in our previous study [19].
Then, the nano-Al and WO3 particles with different molar ratios were added into the dispersing agent of isopropyl alcohol, PEG-1000, and polyethyleneimine (volume ratio = 100:2:1), and the corresponding stable suspension was obtained after ultrasonic process of 200 W for 0.5 h at room temperature. Before EAT, nickel substrates with 1 × 2 × 4 cm3 as working electrode and counter electrode were polished using sandpapers with different mesh numbers (100#–1000#), followed by alkaline washing using a mixture of sodium hydroxide and sodium carbonate, ultrasonic cleaning by ethanol and deionized water, and vacuum drying at 353.15 K, respectively. Two treated electrodes were perpendicularly inserted into stable suspension with a distance of 1.0 cm during EAT process which was conducted under a comparatively low field strength of 20 V/mm. After EAT process for 0.2 h at room temperature, the nano-Al@WO3 energetic films deposited on working electrode was removed to a vacuum drying oven at 378.15 K for 1.0 h. In final, the target energetic coating was obtained after cooling to ~ 398.15 K naturally. Each experiment was repeated three times for high accuracy.
2.3 Material characterization
The macro/micromorphologies, element distribution, and phase composition of the nano-Al@WO3 energetic coatings were analyzed by a field emission scanning electron microscope (FESEM; Auriga, Zeiss, Germany) equipped with energy-dispersive X-ray spectroscopy (EDX) and X-ray diffractometer (XRD-6000, Shimadzu, Japan) with a scanning rate of 5°/min, respectively. Atomic absorption spectroscopy (AAS, 180-80, Exeter Analytical) was used to calculate the mole ratio of reactants in the obtained energetic coating. The heat release of product was measured by differential scanning calorimetry (DSC) (NETZSCH, STA449F3, Germany) carried out at a low heating rate of 15 K/min under Ar flow (99.999%).
3 Results and discussion
The XRD technique was used to analyze the crystal structures of the nano-Al@WO3 energetic coatings, which are shown in Fig. 2. Almost all sharp diffraction peaks were marked, indicating a high crystallinity. Clearly, the XRD pattern of EAT energetic coating was completely indexed as Al (JCPDS card No. 04-0787) with the special space group of Fm-3 m (225) with constants of a = b = c = 4.049Å and axial angle of α = β = γ = 9°, and WO3 (JCPDS No. 20-1323; P-1(2)) with the corresponding crystal (cell) size of a = 7.3Å, b = 7.52Å, c = 7.69Å, and axial angle of α = 88.83°, β = 90.91°, and γ = 90.93°. In addition, there were scarcely any other obvious diffraction peaks (e.g., Al2O3, W), which demonstrated that product possessed high pureness, and no redox reaction occurred during the EAT process. These results not only indicated the presence of both nano-Al and WO3 in the deposit, but also the successful EAT of nano-Al@WO3 energetic coatings. Furthermore, the isopropyl alcohol, PEG-1000, and polyethyleneimine mixture also turns out to be a suitable dispersion medium for the design of EAT of nano-Al@WO3 energetic system.
The electrophoretic nano-Al@WO3 energetic sample coatings prepared in optimized suspension are shown in Fig. 3a. It can be seen that a gray energetic coating with smooth and uniform micromorphology was successfully prepared by EAT based on metallic substrate, and there are almost no lumpy areas or local cracks and delamination, demonstrating the structural superiority of product. This result also indicated that the obtained nano-Al@WO3 energetic deposits have sufficient quality in terms of adhesion to the substrate and structural integrity. Further, Fig. 3b and c displays the FESEM microstructure image with different magnifications of product. Clearly, the surface of target energetic coating was fairly evenly distributed with few reunion areas in lower-resolution FESEM image of product in Fig. 3b. Moreover, the particle size of nano-Al or WO3 in deposited coating was nanoscale with a diameter of ~ 50nm in the higher-resolution FESEM image (Fig. 3c), which can largely enhance the contact area of fuel (nano-Al) and oxidizer (WO3) to improve the sufficiency of the exothermic reaction of obtained energetic coating [16]. It is worth mentioning that there are lots of tiny gaps among nanoparticles (Fig. 3c) as indispensable heat flow channels during heat-release process and thereby increases the heat output capacity of product.
In addition, the elemental compositions in the nano-Al@WO3 energetic coatings were investigated by the EDX analysis (seen in Fig. 4). The typical elemental mapping of product based on the top view FESEM image (yellow virtual box area in Fig. 3b) is displayed in Fig. 4. The elemental mapping showed that Al, W, and O were distributed evenly, indicating the homogeneous mixing of nano-Al and WO3 in product, which is consistent with the XRD analysis. Notably, there are few slight or obvious agglomeration of nanoparticles. Moreover, all expected EDX spectrum peaks of Al, W, and O were obviously observed in Fig. 4, which also demonstrated that the desirable compositional homogeneity in an electrophoretic nano-Al@WO3 energetic coating could be achieved.
EDX spectrum and element mapping images of the target energetic coating based on FESEM top view image of product (yellow virtual box area in Fig. 3b)
The component proportion of target coating is essential to the heat-release performance. Thus, EDX and AAS techniques were used to analyze the mole ratio of all elements, and the corresponding results are shown in Fig. 5. Clearly, for EDX analysis based on Fig. 4, the mole ratio of Al, W, and O in the EDX mapping region in Fig. 4 is 2.1:0.95:2.93 (blue bar chart in Fig. 5a), which was close to the theoretical reaction ratio of product (2.0:1.0:3.0, \(2{\text{Al}} + {\text{WO}}_{3} \to {\text{Al}}_{2} {\text{O}}_{3} + {\text{W}} + {\text{Q}}_{{{\text{Heat - release}}}}\)). Moreover, it is worth noting that the results of elemental molar ratio of sample by AAS analysis were similar to that by EDX technique and theoretical value (green bar chart in Fig. 5a), which provided the solid component foundation of product to release violent energy. Further, two other random regions were used to verify the reliability of uniform distribution of components in nano-Al@WO3 energetic coatings (Fig. 5b and c). It can be seen that the differences between test results were minimal among the elemental molar ratio of Al, W, and O in three random regions analyzed by EDX and AAS technique, respectively, indicating the structural superiority of product by EAT.
Exploration of exothermic performance is necessary to high-energy materials, including nano-Al@WO3 energetic system [20,21,22]. The DSC is a common technique used to analyze heat-release process of product, and the corresponding DSC curve measured in Ar atmosphere is clearly displayed in Fig. 6. It can be seen that with the temperature increase, the heat release (mW/mg) basically increased at first and reached to the peak value at ~ 800 °C, and then decreased sharply as the temperature continued to increase. It is worth mentioning that there was a small endothermic peak marked by green rectangle (Fig. 6) at ~ 660 °C because of the melt process of Al [23]. The main obvious exothermic peak was due to the drastic heat release or thermite reaction between Al and WO3 in nano-Al@WO3 coatings [9, 24, 25]. After a fitting calculation, the output of heat of the target energetic system fabricated by EAT technique can reach up to ~ 2400 J/g that was approximately 80% of theoretical value, demonstrating the well sufficient heat exposure process and exhibiting wide potential application in fields of microelectronics, military, etc.
Superiority of EAT in designing nano-Al@WO3 energetic coating is also verified by adjusting the EAT process parameters (e.g., EAT time, the distance of electrodes, EAT temperature) to analyze the heat process stability of product. Figure 7a displays the relationship of heat release of product and the distance (D) between working electrode and counter electrode. It can be seen that the heat release increased slowly with the “D” increase from 4 to ~ 10 mm and then kept almost stable, which indicated that the exothermic performance is hardly influenced by the value of “D”. The effect of the EAT time and temperature on the output of heat of sample is shown in Fig. 7b and c. Clearly, as the EAT increased, there was a small fluctuation in the value of heat release, whose variation range was < 5%, which also indicated the good stability of particle concentration in optimized suspension and the highly controllable EAT process used, combined with wide potential applications for designing other kinds of promising micro/nano-energetic systems.
4 Conclusion
In summary, the novel nano-Al@WO3 energetic coating with even distribution has been successfully fabricated using one-step electrophoretic assembly technique under mild conditions. The optimized suspension was the mixture of isopropyl alcohol, PEG-1000, and polyethyleneimine. The product possessed a high crystallinity and purity which was demonstrated by XRD pattern analysis. FESEM and EDX results showed that WO3 nanoparticles relatively evenly distributed among fuel (nano-Al particles) with few agglomerations, and the molar ratio of reagents was determined by EDX and AAS techniques. In addition, the violent exothermic process of product was investigated by DSC curve and the total output of heat was as high as ~ 2400 J/g with the promising stability, showing wide applications in fields of military, trigger, etc.
References
Y.G. Xu, Q. Wang, C. Shen, Q.H. Lin, P.C. Wang, M. Lu, A series of energetic metal pentazolate hydrates. Nature 549, 78 (2017)
Z.X. Song, M.M. Jin, M. Xian, C.D. Wang, Peptide-driven assembly of Al/CuO energetic nanocomposite material. Chem. Eng. J. 388, 124225 (2020)
J. Dai, J. Xu, F. Wang, Y. Tai, Y. Shen, R. Shen, Y. Ye, Facile formation of nitrocellulose-coated Al/Bi2O3 nanothermites with excellent energy output and improved electrostatic discharge safety. Mater. Des. 143, 93 (2018)
L. Marín, C.E. Nanayakkara, J.-F. Veyan, B. Warot-Fonrose, S. Joulié, A. Estève, C. Tenailleau, Y.J. Chabal, C. Rossi, Enhancing the reactivity of Al/CuO nanolaminates by Cu incorporation at the interfaces. ACS Appl. Mater. Interfaces 7, 11713 (2015)
G.Q. Jian, J.Y. Feng, R.J. Jacob, G.C. Egan, M.R. Zachariah, Super-reactive nanoenergetic gas generators based on periodate salts. Angew. Chem. Int. Ed. 52, 9743 (2013)
D.J. Shin, W.D. Kim, S. Lee, D.C. Lee, Nanothermite of Al nanoparticles and three-dimensionally ordered macroporous CuO: mechanistic insight into oxidation during thermite reaction. Combust. Flame 189, 87 (2018)
W. He, P.-J. Liu, G.-Q. He, M. Gozin, Q.-L. Yan, Highly reactive metastable intermixed composites (MICs): preparation and characterization. Adv. Mater. 30, 1706293 (2018)
P. Gibot, A. Bach, L. Vidal, S. Fabien, G. Roger, S. Denis, Safer and performing energetic materials based on polyaniline-doped nanocomposites. J. Energy Mater. 35, 136 (2017)
G.H. Fan, Q.W. Wang, L. Geng, G.S. Wang, Y.C. Feng, Preparation and characterization of aluminum matrix composites based on Al-WO3 system. J. Alloys Compd. 545, 130 (2012)
V.E. Sanders, B.W. Asay, T.J. Foley, B.C. Tappan, A.N. Pacheco, S.F. Son, Reaction propagation of four nanoscale energetic composites (Al/MoO3, Al/WO3, Al/CuO, and Bi2O3). J. Propul. Power 4, 707 (2007)
A. Bach, P. Gibot, L. Vidal, R. Gadiou, Modulation of the reactivity of a WO3/Al energetic material with graphitized carbon black as additive. J. Energy Mater. 33, 260 (2015)
S. Maeda, Q.H. Qu, M.J. Robertson, G. Skiniotis, B.K. Kobilka, Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552 (2019)
D.X. Zhang, X. Wang, X.F. Peng, Q.Y. Wang, Q. Xiang, Kinetics of electrophoretic deposition of nano-Co3O4 coating. J. Mater. Sci.-Mater. Electron. 30, 8967 (2019)
C. Timm, C.M. Niemeyer, Assembly and purification of enzyme-functionalized DNA origami structures. Angew. Chem. Int. Ed. 54, 6745 (2015)
F. Praetorius, B. Kick, K.L. Behler, M.N. Honemann, D. Weuster-Botz, H. Dietz, Biotechnological mass production of DNA origami. Nature 552, 84 (2017)
X. Guo, C. Lai, X. Jiang, W. Mi, Y. Yin, X. Li, Y. Shu, Remarkably facile fabrication of extremely superhydrophobic high-energy binary composite with ultralong lifespan. Chem. Eng. J. 335, 843 (2018)
K.T. Sullivan, C. Zhu, E.B. Duoss, A.E. Gash, D.B. Kolesky, J.D. Kuntz, J.A. Lewis, C.M. Spadaccini, Controlling material reactivity using architecture. Adv. Mater. 28, 1934 (2016)
X.G. Guo, X.M. Li, Z.B. Wei, X.M. Li, L.N. Niu, Rapid fabrication and characterization of superhydrophobic tri-dimensional Ni/Al coatings. Appl. Surf. Sci. 387, 8 (2016)
X.G. Guo, T.T. Liang, B.F. Yuan, J. Wang, Q. Sun, Controllably facile design of electrophoretic-induced film-forming of nano tungsten oxide (VI) and their anti-wetting functionalization. Nanotechnology 31, 505603 (2020)
X.X. Ma, Y. Zhu, S.X. Cheng, H.X. Zheng, Y.S. Liu, Z.Q. Qiao, G.C. Yang, K.L. Zhang, Energetic composites based on nano-Al and energetic coordination polymers (ECPs): the “father-son” effect of ECPs. Chem. Eng. J. 392, 123719 (2020)
J.Y. Zhou, L.L. Ding, W.H. Tang, T.W. Ran, Experimental study of mechanical properties and impact-induced reaction characteristics of PTFE/Al/CuO reactive materials. Materials 13, 66 (2020)
H.Y. Wang, J.P. Shen, D.J. Kline, N. Eckman, N.R. Agrawal, T. Wu, P. Wang, M.R. Zachariah, Direct writing of a 90 wt% particle loading nanothermite. Adv. Mater. 31, 1806575 (2019)
P. Yu, C.-J. Deng, N.-G. Ma, D.H.L. Ng, A new method of producing uniformly distributed alumina particles in Al-based metal matrix composite. Mater. Lett. 58, 679 (2004)
S. Valliappan, J. Swiatkiewicz, J.A. Puszynski, Reactivity of aluminum nanopowders with metal oxides. Powder Technol. 156, 164 (2005)
P. Gibot, Q. Miesch, A. Bach, F. Schnell, R. Gadiou, D. Spitzer, Mechanical desensitization of an Al/WO3 nanothermite by means of carbonaceous coatings derived from carbohydrates. J. Carbon Res. 5, 37 (2015)
Acknowledgements
This research was funded by the National Natural Science Foundation of China (No. 21805014), the Natural Science Foundation of Chongqing (No. cstc2019jcyj-msxmX0675), and Yangtze Normal University (No. 2018QNRC10).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, X., Liang, T., Bao, H. et al. Novel electrophoretic assembly design of nano-aluminum@tungsten trioxide (nano-Al@WO3) energetic coating with controllable exothermic performance. J Mater Sci: Mater Electron 32, 15242–15250 (2021). https://doi.org/10.1007/s10854-021-06075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06075-2