Abstract
Gd2O3-doped glasses in the B2O3–CaO–Na2O–SrO–P2O5 system were synthesized via melt annealing route and characterized through physical properties. With the replacement of CaO by Gd2O3, the measured values of the density (ds), Gd3+ ions concentration (N), packing density (Pd), oxygen packing density (OPD), Vickers’s hardness (HV), and field strength (F) of the synthesized samples increased, whereas the molar volume (Vm), free volume (Vf), polaron radius (rp), average boron–boron distance (dB–B), and inter-nuclear distance (ri) decreased. The glassy nature of the synthesized samples is confirmed by the X-ray diffraction patterns. The change in the coordination number of boron and the different B–O vibrational bands with the incorporation of gadolinium ions in the investigated glass samples were examined by Raman and FTIR spectroscopy, which supported the presence of BO3, BO4, and GdO4 groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Borate glasses containing rare-earth oxide are among the most important materials which have been invented since ancient time. They were considered as useful materials in different field applications [1,2,3]. Due to the potential practical applications in advanced laser materials [4], optical, memory devices [5], and biomaterials glass fields [6], the study of the borate glass is of great interest for basic research. In recent years, borate-based glasses are very important because of their interesting chemical and physical properties such as their significantly lower melting temperature, low viscosity, good mechanical stability, low chemical durability, attainability at low cost, and high visible light transparency [7,8,9]. It is known that in silicate glass, silicon is present in SiO4 [10]. In contrast to silicate glass, vitreous borate glasses contain a mixture of triangles units BO3 and tetrahedral units BO4 depending on the composition of the glass [11]. Incorporation of the network-modifying cations such as (CaO, SrO, and Na2O) into the borate matrix glass is suggested to modify the network structure and change the environment of the glass network [3, 6, 12, 13]. These modifiers will either be used to break one of the three bonds between boroxol rings to convert boron from a three-coordinated (BO3) unit to a four-coordinated (BO4) unit or to create non-bridging NBOs depending on the concentrations of the B2O3 as a former and these modifiers [14]. Depending on the type and amount of the modifier oxide, the maxima of the fraction of boron in tetrahedral coordination (N4) occurs around 35–45 mol% [15]. It has been reported that the transformation between BO3 and BO4 boron groups as a function of the composition has a major influence on properties of glass such as its density, hardness, and molar volume (Vm) [16]. Strontium is an alkaline earth metal which falls after calcium in the periodic table and shares many similar properties [17]. The presence of SrO as well as CaO in the glass compositions causes increase in the density, structural rigidity [18], and enhances the bioactive properties of borate glasses [19]. Nowadays, transition metal, lanthedate and, rare-earth oxide doped glass and glass–ceramic are of increasing interest in various types of applications such as optical [20], electrical [21], and biomedical application [22]. The rare-earth oxide is used as a glass dopant to probe the glass environment’s structure due to its sharp energy levels and the modifications of the energy level of the modifier ions structure by the glassy environment [23]. In general, lanthanides are stable as trivalent ions in most glass synthesized using the conventional melt technique. As a classic lanthanide element, studies on Gd2O3 containing borate glasses are limited. The structural roles of the alkali and alkaline earth oxides are well known in borate glass, but the role of Gd2O3 in such is not well known especially in bioborate glass systems. Therefore, our study is to evaluate the structural variation induced by Gd2O3 and identify its influence on the physical properties of borate-based glass.
2 Experimental work and techniques
2.1 Sample preparation
Glass samples were synthesized by using high-purity analytical grade chemical as reserved. Gadolinium oxide (Gd2O3, Sigma-Aldrich 99.9%) was introduced as such, B2O3 was introduced as H3BO3 (Sigma-Aldrich, 99.5%), Ammonium dihydrogen orthophosphate (NH4H2PO4, Sigma-Aldrich 98%) was used as the source for P2O5, SrO was introduced as SrCO3 (98.5%, Sigma-Aldrich, Germany), CaO and Na2O supplied by Aldrich company were also introduced as CaCO3 (99%) and Na2CO3 (99%), respectively, which were used as starting materials to synthesize glass samples with composition 42.7B2O3–(23.5−x)CaO–26Na2O–5SrO–2.8P2O5−xGd2O3, where 0 < x < 0.5 mol% (Table 1).
The electronic beam balance technique with an accuracy of 0.0001 g was used to weigh the chemical powders, and they were mixed with appropriate amounts of raw materials. The batches were put into the porcelain crucibles and then melted in the programmable electrical furnace at 1070–1150 °C for 60 min. After occasionally stirring the molten liquid for many times to ensure homogenizing the melt and to obtain bubble-free samples, they were cast into pre-heated (at 300 °C) stainless steel plates of the required dimensions and subsequently annealed for 1 h and then cooled slowly to room temperature to remove the thermal and internal stress from the samples. The glass samples in this research are colorless.
2.2 Spectroscopic investigations
2.2.1 X-ray spectroscopy
X-ray diffraction diffractogram of the synthesized samples was obtained by a Bruker Axs-D8 technique using a source CuKα radiation (λCuKα = 0.1540600 nm). X-ray diffractometer operates at 40 kV as accelerating voltage and 30 mA current in the intervals of 0.02°, over a 2 theta ranging from 4° to 70° using a time of 0.4 s. Data were fitted with an Advantech super speed position-sensitive detector.
2.2.2 FTIR spectroscopy
FTIR spectra were obtained using FTIR spectroscopy (Mattson 5000, USA) for all the observed glasses in the wavenumber range 400–4000 cm−1 at 2.0 cm−1 resolution to perform the function group of these samples. Phosphate, borate, and the hydroxyl groups were characterized. To obtain good quality spectra, the glassy specimens were grinded to powder and dispersed in KBr in the ratio 1:100. The mixture was subjected to a constant pressure of 5 ton cm−2. Clear transparent discs were obtained and measured immediately after preparation. The dark current noises and the background of the obtained IR spectra were corrected using two-point baseline correction before fitting.
2.2.3 Raman mapping spectroscopy
Raman spectra of the undoped glass and glass containing Gd2O3 were recorded using a Renishaw in Via Raman microscope with a laser line at 632.8 nm wavelength to excite the specimen. It is operating at 500 mW and for each scan eight acquisitions were taken.
2.2.4 Physical properties
2.2.4.1 Density and molar volume measurements
Archimedes’ principle was used to measure the glass density (ds) at room temperature (30 °C) for all prepared bulk samples using Xylene as an immersion liquid (density = 0.865 g cm−3). A digital balance (Mettler-Toledo, with error ± 0.0001 g) was used to weigh the bulk solid in air (WSA) as well as in the solvent (WSL), and the ds is calculated according to the following equation [24]:
where ds is the density of the sample, WSA and WSL are the weights of the sample in the air and liquid, respectively, and dL is the density of the Xylene. The density measurements were carried out on three samples of each glass, and the average value was calculated. Density values are precise to ± 0.002 g cm−3.
The Vm is defined as the volume of the substance containing one mol%. Vm of the prepared glass samples were calculated from the obtained density data using the formula [24]:
where Mi is the molecular mass for a component i, ni is the molar ratio, and ds is the density of the sample.
2.2.4.2 Free volume (V f) and packing density (Pd)
The Vf was defined as the unoccupied space which exists between molecules, and it is used to explain the mobility of the molecular within the network. The Vf is given in the equation:
where xi is the molar ratio of the samples and Vi is the Vm of each component.
Pd was defined as the ratio between the minimum fraction of volume occupied by the ions and the corresponding effective volume of glass [25]. Pd was obtained using the next formula [25]:
OPD is considered as a measure of packing oxide network tightening of the prepared glass samples and can be evaluated by applying the relation [26]:
where M is the average molecular weight and n is the number of oxygen atoms per formula unit.
The effect of dopant concentration in the glass matrix can be identified by the average boron–boron separation according to the following relation [27]:
where \(V_{{\text{m}}}^{{\text{b}}}\) is the molar volume of boron atoms and is given by
where XB is the mole fraction of B2O3 content.
2.2.4.3 Ion concentration (N)
The Gd3+ ions concentration (N) is calculated using the equation [28]
A polaron was defined as a quasi-particle which is used to describe the interaction between the electrons and ions and also used to understand the interaction between the electrons and atoms in the solid materials. The polaron radius (rp) in (Å) can be evaluated depending on the value obtained of N, using the following equation [29]:
The inter-nuclear distance (ri) was calculated according to the next expression [30]:
The field strength (F) can be evaluated using the equations [30]:
where Z the molar mass of the rare earth (gadolinium oxide).
2.2.4.4 Microhardness measurements (HV)
The hardness of the specimens was determined using a digital Vickers microscope model (FM-7, Future-Tech Corporation, Kawasaki-Japan). The Vickers’s hardness (HV) test was performed under 50 g load and 15 s of dwell time. The sample surface was polished, and twelve indentations were made on the surface of each specimen at room temperature and the average value of the diagonal was used to calculate HV from the following equation [31]:
where F is the applied indentation load in kgf, and d is the arithmetic mean of the two measured indentation diagonals d1 and d2 in mm. The applied indentation load was calculated using the following formula:
where n is Meyer’s index number, K is the substance constant, n value was obtained from the plot of logF versus logd since the slope of the line yields represent the value of n, which indicates whether the substance is hard or soft.
3 Result and dissection
3.1 X-ray analysis
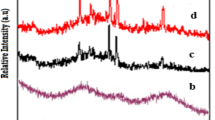
The nature of the glassy materials can be understood using X-ray powder diffraction, but this approach is particularly useful for the analysis of the glassy materials network. Figure 1 shows the X-ray diffraction spectra of the B2O3–CaO–Na2O–SrO–P2O5 glass system containing different concentrations of Gd2O3. Broadband appears in the wide range (2θ = 25°–35°) and showed no identifiable diffraction peaks indicating that Gd2O3 containing glass has amorphous nature.
3.2 Vibrational spectroscopy
FTIR and Raman spectroscopies are powerful tools for structural analysis and are used as complementary techniques. They used with advantage to understand the structure and environment of the matrix, although they differ slightly from the working principle. The Raman spectra depend on the change of the polarizability. On the other hand, the bands arise from the polarizability change due to the scattering of light by the vibrating molecules, whereas the IR bands arise from the change in the dipole moments as a result of the absorption of light by molecules vibrations. A view of transitions are forbidden in IR spectroscopy, but they are allowed in Raman spectra [32]. These two techniques have been employed to know the functional groups present in the glass samples.
3.2.1 FTIR interpretation
Figure 2 demonstrates FTIR measurements in a region 400–4000 cm−1 for the prepared glass samples. FTIR spectrum reported some bands at around 570, 708, 1022, 1220, and 1406 cm−1. The bands at about 1220–1406 cm−1 are attributed to the vibration of the various borate groups (B–O bond stretch of the BO3 units) and related to B–O–B bending vibrations [33]. The band at about 1022 cm−1 is attributed to the B–O bonding stretch of BO4 units [34]. The small band at 708 cm−1 can be ascribed to the symmetric strength vibration of BO3 [34]. The peaks within the range 400–600 cm−1 are assumed by some authors [34, 35] to represent vibrations of modifier cations (Na+, Ca2+) and bending mode of PO43− in these glasses [35]. As we have observed, the band at around 570 cm−1 described B–O–Gd, and Gd–O–Gd bending modes and GdO4. The assignments for each FTIR bands are recorded in Table 2.
Peak fit program was used to de-convolute the FTIR spectra of all the prepared samples, and the de-convolution parameters are given in Table 4 in Appendix. The FTIR spectra were corrected from the dark current noises and background using two points of baseline correction before fitting previously discussed [36, 37]. Figure 3a, b displays the FTIR de-convolution, in Gaussian band, and the residual graph for 42.7B2O3–23.4CaO–26Na2O–5SrO–2.8P2O5–0.1Gd2O3 sample, respectively, as an example. It was found that the difference between the experimental and simulated curves is less than 0.05% as can be seen in Fig. 3b. And then BO3 and BO4 peaks areas were integrated to calculate the N4 using the next formula:
Figure 4 displays the N4 as a function of Gd2O3 content to estimate the Gd2O3 effect on the change of the relative population of tetrahedral units BO4 and triangle units BO3. It is observed that N4 values varied between 0.47980 and 0.51502 according to the Gd2O3 concentration. It would appear that the addition of Gd2O3 tends to decrease BO4 units.
3.2.2 Raman spectroscopy analysis
Normalized Raman spectra of the studied undoped glass and glasses containing different concentrations of Gd2O3 are shown in Fig. 5. Raman spectra consist of three major regions to clarify the effects of Gd2O3 in the glass network. The first is the low-wavenumber region extending from 400 to 600 cm−1. The second is a mid-wavenumber region extending from 600 to 1100 cm−1. Besides, the high wavenumber region falls between 1100 and 1600 cm−1. Previous studies indicate that the Raman spectrum of the vitreous B2O3 is dominated by a strongly polarized intense band at 810 cm−1, which evidences that B2O3 glass consists mainly of boroxol rings, the band at 810 cm−1 being the characteristic feature [38,39,40]. But when the alkali and alkaline earth oxide were added, several changes occurred in the network of borate glass [38]. For example, the band at 810 cm−1 disappears in almost all of alkali and alkali earth borate glass for > 20 mol% [38], such as in the present work. The Raman spectrum of the present work (Fig. 5) does not contain a band at around 810 cm−1, which indicates that most of the boroxol rings were converted to diborate (in which BO3 and BO4 are equal), triborate, and dipentaborate groups. The most notable is that, in the mid-frequency region (600–1100 cm−1) of the measured spectra, the Raman bands displayed a strong peak due to B−O vibrations, since the glasses under investigation are low in Gd2O3 and strong in B2O3 concentrations. And the sharpening of this peak in the free and containing Gd2O3 glass correlates usually to the asymmetric vibrations of pentaborate, tetraborate, and connected to the diborate groups (947 cm−1) [39, 40]. The band at about 761 cm−1 is attributed to the six-membered rings with BO4− units [36]. The low-frequency broadband centered at about 500 cm−1 is assigned to the loose diborate & BO4 groups due to the higher alkali oxide content [40] and typically arises from B−O−B bending modes, Gd−O−Gd and B−O−Gd stretching vibration in units of BO4 and GdO4. The band in the range of 400–600 cm−1 is attributed to the bridging anion mode of heavy metal oxide [41]. The formation of GdO4 is depicted by the band at 500 cm−1 due to the vibration of Gd4−O−Gd4.
With growing Gd2O3 content, the relative area under the bands and the band’s amplitude are increased due to the vibrations of GdO4 tetrahedral units containing bridging oxygen (BO) and non-bridging oxygen (NBO). Finally, the Raman spectra include a medium band at about 1487 cm−1 which appears at a higher frequency area and is assigned to the vibration of B−O− the BO3 units, occurring in a large borate network. This band contains contributions from symmetrical triangle boron units [40]. From the above discussion, it is found that the Raman and FTIR results supported the presence of BO3, BO4, and GdO4 groups. Peaks’ position of the Raman spectra is recorded in Table 2.
3.3 Physical properties
3.3.1 Density and molar volume
Density and Vm of undoped glass and glasses containing gadolinium oxide are given in Table 3 and Fig. 6. It is observed that the density of the investigated samples increases from 2.64 to 2.78 g cm−3 due to the increase of Gd2O3 mol% (from 0.0 to 0.5 mol%).
This increase can be explained based on the lower atomic weight of CaO (atomic mass, ZCaO = 56.08 g mol−1) as compared to Gd2O3 (atomic mass, ZGd2O3 = 362.4982 g mol−1). Moreover, the density of Gd2O3 (7.41 g cm−3) is higher than CaO (3.34 g cm−3), leading to an increase in the density of the prepared samples.
Figure 7 shows that Pd displays a slight increase with the addition of Gd2O3 content due to the increase in the number of the oxygen atoms resulting from higher atomic weight of Gd2O3 [42]. It is observed also from Fig. 6 and Table 3 that the Vm of the investigated glass samples is found to decrease from 25.77 to 25.07 cm3 mol−1. The ionic radius of Gd (0.938 Å) is smaller than that of Ca (1 Å), therefore the decrease of Vm can be explained based on the substitution CaO by Gd2O3 causing shrinkage of the glass network. The increase in the density values will lead to a decrease in the Vm due to the inverse relationship between them [42, 43]. Hence, the compactness of the synthesized glass will increase [44]. Besides, the Vm is affected also by the decreasing Vf. The substitution of CaO with Gd2O3 has an effective influence on the inter-atomic spacing between the atoms or in bond length. The variation in the Vm was affected by the rearrangement of lattice within the glass network, the number of bonds per unit volume [45], and the stretching force constant of the bonds inside the glassy [45].
3.3.2 Ion concentration, polaron radius, and field strength
The calculated values of physical parameters such as Gd3+ ions concentration, rp, ri, F, and OPD of the prepared glass samples were recorded in Table 3 and illustrated as functions of gadolinium concentration in Fig. 8a, b. They reveal that values of both Gd ions concentration and F are increased linearly according to the increase of the Gd2O3 content, while the values of both rp and ri are decreased, which is a logical result where the atomic radius of gadolinium (238 pm) is larger than that of the calcium (231 pm). The decreasing of the rp and ri and the increasing values of F indicator to the stronger bond between Gd–O, which in turn producing stronger filed around Gd3+ ions. It can be also observed from Table 3, that the decreases of the average boron–boron distance, < dB–B > , (nm) with increasing Gd2O3 content denotes the increase in compactness of the glass network and also supports the increments in the glass density as discussed above. Increasing ion concentrations lead to decreases in rp and inter-nuclear distance. The average molar mass of the synthesized samples also increases with the increasing Gd2O3 content. One of the important physical parameters is OPD, which provides an insight into the structure of the prepared glass samples. It is observed that the OPD values increase with increasing Gd2O3 content, this result is in agreement with density and Vm results. As a result, the addition of the Gd2O3 makes the glass network high and tightly packed.
3.3.3 Hardness of the prepared glass samples
Figure 9 represents the HV values as a function of the Gd2O3 content. As noted below, the values of HV increase with increasing Gd2O3 content, indicating the formation of a rigid structure which makes the glass harder. Cations that have smaller radii and high F are high anf strongly attracted to the surrounding structural units of borate units. Therefore, it is worthy to note that the smaller radii and high F of Gd cations can affect the hardness number of borate glass. The increase in hardness and density is due to the higher molecular weight of Gd2O3 (362.4982 g mol−1) compared with CaO (56.08 g mol−1), and coupled with high F. The presence of GdO4, BO3, & BO4 groups in the glass network causes an increase in the connectivity of the structure which leads to an increase in microhardness values. Therefore, the investigated samples become more compact (more tightly packed).
4 Conclusion
Borate glasses containing different concentrations of Gd2O3 have been characterized using several techniques. XRD patterns of the investigated samples revealed amorphous nature. The changes in the FTIR and Raman spectra and physical properties due to the addition of Gd2O3 contents result from the changes in bonding configuration, mainly Gd–O–B bonds and stretching vibrations of BO4, GdO4, and BO3 units in the glass structure. The density values increase with increasing the heavier Gd2O3 at the expense of lighter CaO content. The result of the Raman spectra supported the presence of GdO4, BO3, & BO4. The density, Pd, and microhardness of the prepared samples were found to be increased with Gd2O3 content, while the Vm and Vf were found to decrease demonstrating an opposite trend. The presence of GdO4, BO3, & BO4 groups in the glass network causes an increase in the connectivity of the structure which leads to an increase in microhardness values. Raman spectra revealed that the borate glasses network is affected by the addition Gd2O3 content.
Code availability
Not applicable.
References
E.I. Kamitsos, G.D. Chryssikos, Borate glass structure by Raman and infrared spectroscopies. J. Mol. Struct. 247, 1–16 (1991)
M. Farouk, A. Samir, F. Metawe, M. Elokr, Optical absorption and structural studies of bismuth borate glasses containing Er3+ ions. J. Non. Cryst. Solids 371, 14–21 (2013)
A.K. Yadav, P. Singh, A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 5(83), 67583–67609 (2015)
V. Venkatramu, D. Navarro-Urrios, P. Babu, C.K. Jayasankar, V. Lavin, Fluorescence line narrowing spectral studies of Eu3+–doped lead borate glass. J. Non. Cryst. Solids 351(10–11), 929–935 (2005)
B. Sumalatha, I. Omkaram, T.R. Rao, C.L. Raju, The structural, optical and magnetic parameter of manganese doped strontium zinc borate glasses. Phys. B Condens. Matter 411, 99–105 (2013)
A.M. Abdelghany, M.A. Ouis, M.A. Azooz, H.A. ElBatal, G.T. El-Bassyouni, Role of SrO on the bioactivity behavior of some ternary borate glasses and their glass ceramic derivatives. Spectrochim. Acta. Part A. 152, 126–133 (2016)
M. Bengisu, Borate glasses for scientific and industrial applications: a review. J. Mater. Sci. 51(5), 2199–2242 (2016)
M. Mariyappan, K. Marimuthu, M.I. Sayyed, M.G. Dong, U. Kara, Effect Bi2O3 on the physical, structural and radiation shielding properties of Er3+ ions doped bismuth sodium fluoroborate glasses. J. Non. Cryst. Solids 499, 75–85 (2018)
S.B. Kolavekar, N.H. Ayachit, G. Jagannath, K. NagaKrishnakanth, S.V. Rao, Optical, structural and Near-IR NLO properties of gold nanoparticles doped sodium zinc borate glasses. Opt. Mater. (Amst) 83, 34–42 (2018)
K.H. Karlsson, K. Fröberg, Structural units in silicate glasses. Chem. Geol. 62(1–2), 1–5 (1987)
P. Naresh et al., Modifier role of ZnO on the structural and transport properties of lithium boro tellurite glasses. J. Non. Cryst. Solids 514, 35–45 (2019)
G. Sangeetha, K.C. Sekhar, A. Hameed, G. Ramadevudu, M.N. Chary, M. Shareefuddin, Influence of CaO on the structure of zinc sodium tetra borate glasses containing Cu2+ ions. J. Non. Cryst. Solids 563, 120784 (2021)
G. El-Damrawi, K. Abd-El-Nour, R.M. Ramadan, Structural and dielectric studies on Na2O–PbO–SiO2 glasses. SILICON 11(1), 495–500 (2019)
Y. Zhou, H. Li, K. Lin, W. Zhai, W. Gu, J. Chang, Effect of heat treatment on the properties of SiO2–CaO–MgO–P2O5 bioactive glasses. J. Mater. Sci. Mater. Med. 23(9), 2101–2108 (2012)
W.C. Lepry, S.N. Nazhat, The anomaly in bioactive sol–gel borate glasses. Mater. Adv. 1(5), 1371–1381 (2020)
M.S. Gaafar et al., Role of neodymium on some acoustic and physical properties of Bi2O3–B2O3–SrO glasses. J. Mater. Res. Technol. 9(4), 7252–7261 (2020)
U. Patel et al., In vitro cellular testing of strontium/calcium substituted phosphate glass discs and microspheres shows potential for bone regeneration. J. Tissue Eng. Regen. Med. 13(3), 396–405 (2019)
R. Divina, K.A. Naseer, K. Marimuthu, Y.S.M. Alajerami, M.S. Al-Buriahi, Effect of different modifier oxides on the synthesis, structural, optical, and gamma/beta shielding properties of bismuth lead borate glasses doped with europium. J. Mater. Sci. Mater. Electron (2020). https://doi.org/10.1007/s10854-020-04662-3
H. Tripathi, C. Rath, A.S. Kumar, P.P. Manna, S.P. Singh, Structural, physicomechanical and in-vitro bioactivity studies on SiO2–CaO–P2O5–SrO–Al2O3 bioactive glasses. Mater. Sci. Eng. 94, 279–290 (2019)
N. Elkhoshkhany, R. Abbas, R. El-Mallawany, A.J. Fraih, Optical properties of quaternary TeO2–ZnO–Nb2O5–Gd2O3 glasses. Ceram. Int. 40(9), 14477–14481 (2014)
H.A. ElBatal et al., In vitro bioactivity behavior of some borophosphate glasses containing dopant of ZnO, CuO or SrO together with their glass-ceramic derivatives and their antimicrobial activity. Silicon 11(1), 197–208 (2019)
B. Samanta, D. Dutta, S. Ghosh, Synthesis and different optical properties of Gd2O3 doped sodium zinc tellurite glasses. Phys. B Condens. Matter 515, 82–88 (2017)
D. Maniu, T. Iliescu, I. Ardelean, I. Bratu, C. Dem, Studies of Borate Vanadate Glasses Using Raman and IR Spectroscopy (Stud. Univ. Babes-Bolyai Phys, Romania, 2001), pp. 366–371
P. Kaur, S. Kaur, G.P. Singh, D.P. Singh, Sm3+ doped lithium aluminoborate glasses for orange-colored visible laser host material. Solid State Commun. 171, 22–25 (2013)
T. Rouxel, Elastic properties of glasses: a multiscale approach. Comptes Rendus Mec. 334(12), 743–753 (2006)
D. Saritha, Y. Markandeya, M. Salagram, M. Vithal, A.K. Singh, G. Bhikshamaiah, Effect of Bi2O3 on physical, optical and structural studies of ZnO–Bi2O3–B2O3 glasses. J. Non. Cryst. Solids 354(52–54), 5573–5579 (2008)
G.P. Singh, S. Kaur, P. Kaur, D.P. Singh, Modification in structural and optical properties of ZnO, CeO2 doped Al2O3–PbO–B2O3 glasses. Phys. B 407(8), 1250–1255 (2012)
M.H.A. Mhareb et al., Impact of Nd3+ ions on physical and optical properties of lithium magnesium borate glass. Opt. Mater. (Amst) 37, 391–397 (2014)
V. Bhatia et al., Mixed transition and rare-earth ion-doped borate glass: structural, optical and thermoluminescence study. J. Mater. Sci. Mater. Electron. 30(1), 677–686 (2019)
S. Karki, C.R. Kesavulu, H.J. Kim, J. Kaewkhao, N. Chanthima, Y. Ruangtaweep, Physical, optical and luminescence properties of B2O3–SiO2–Y2O3–CaO glasses with Sm3+ ions for visible laser applications. J. Lumin. 197, 76–82 (2018)
H. Chandler, Introduction to Hardness Testing (Hardness testing, USA, 1999), pp. 1–13
B. Schrader (ed.), Infrared and Raman Spectroscopy (VCH Publ. Inc., New York, 1995), p. 136
A.M. Abdelghany, Novel method for early investigation of bioactivity in different borate bio-glasses. Spectrochim. Acta. Part A. 100, 120–126 (2013)
S.A. Dalhatu, R. Hussin, K. Deraman, Structural and luminescence properties of Eu3+–doped magnesium sulfide borate glass and crystal. Chinese J. Phys. 54(6), 877–882 (2016)
M. Karabulut, A. Popa, G. Borodi, R. Stefan, An FTIR and ESR study of iron–doped calcium borophosphate glass–ceramics. J. Mol. Struct. 1101, 170–175 (2015)
A.M. Abdelghany, The elusory role of low level doping transition metals in lead silicate glasses. Silicon 2(3), 179–184 (2010)
B. Ashok, K.C. Sekhar, B.S. Chary et al., Physical and structural study of Al2O3–NaBr–B2O3–CuO glasses. Indian J Phys (2021). https://doi.org/10.1007/s12648-021-02048-7
B.N. Meera, J. Ramakrishna, Raman spectral studies of borate glasses. J. Non. Cryst. Solids 159(1–2), 1–21 (1993)
A.A. Osipov, L.M. Osipova, Raman scattering study of barium borate glasses and melts. J. Phys. Chem. Solids 74(7), 971–978 (2013)
A.A. Osipov, L.M. Osipova, B. Hruška, A.A. Osipov, M. Liška, FTIR and Raman spectroscopy studies of ZnO–doped BaO⋅ 2B2O3 glass matrix. Vib. Spectrosc. 103, 102921 (2019)
M.R. Ahmed, B. Ashok, S.K. Ahmmad, A. Hameed, M.N. Chary, M. Shareefuddin, Infrared and Raman spectroscopic studies of Mn2+ ions doped in strontium alumino borate glasses: describes the role of Al2O3. Spectrochim. Acta. Part A. 210, 308–314 (2019)
M.K. Halimah, W.M. Daud, H.A.A. Sidek, A.W. Zaidan, A.S. Zainal, Optical properties of ternary tellurite glasses. Mater. Sci. 28(1), 173–180 (2010)
S. Rada, E. Culea, M. Bosca, M. Culea, P. Pascuta, M. Neumann, Effect of the introduction of gadolinium ions in Boro–tellurite glasses. J. Optoelectron. Adv. Mater. 10(9), 2316–2318 (2008)
Y.B. Saddeek, Elastic properties of Gd3+–doped tellurovanadate glasses using pulse-echo technique. Mater. Chem. Phys. 91(1), 146–153 (2005)
Y. Al-Hadeethi, M.I. Sayyed, Effect of Gd2O3 on the radiation shielding characteristics of Sb2O3–PbO–B2O3–Gd2O3 glass system. Ceram. Int. 46(9), 13768–13773 (2020)
Funding
No funding.
Author information
Authors and Affiliations
Contributions
MAM: conceptualization, methodology, validation, investigation, writing—original draft, writing—review & editing, and visualization. GED: supervision, writing—review & editing. AMA: methodology and formal analysis. MIA: supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Madshal, M.A., El-Damrawi, G., Abdelghany, A.M. et al. Structural studies and physical properties of Gd2O3-doped borate glass. J Mater Sci: Mater Electron 32, 14642–14653 (2021). https://doi.org/10.1007/s10854-021-06022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06022-1