Abstract
The La0.67Ca0.33MnO3 polycrystalline ceramics were prepared by the co-precipitation method with ammonium carbonate as the precipitant and the influence of the sintering temperature on the electrical transport properties of the ceramics were investigated. XRD results show that the ceramic structures obtained are all perovskite structures, which belong to the Pnma space group. Scanning electron microscopy (SEM) photographs demonstrated that the average size of the grains with the sinter temperature increased from 5.78 μm at 1300 °C to 24.04 μm at 1475 °C. Meanwhile, when the sintering temperature reached 1450 °C, the TCR reached the maximal value (32.3%·K−1), which was much larger r than the TCR at 1350 °C. The theoretical model analysis of the data leads to the conclusion that the conductive mechanism of the low-temperature metallic region and the high-temperature insulating region is dominated by the grain boundary scattering effect and the small-polariton hopping (SPH), respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, Manganese-based perovskite materials with CMR effect are another strong correlated material after high-temperature superconductors [1,2,3,4,5]. The general formula is RExAE1-xMnO3 (RE is the rare-earth element, La3+, Nd3+, etc.; AE is the alkali earth element, Ca2+, Sr2+, etc.) while being of extensive attention due to special physical properties, for instance, the colossal magnetoresistance effect (CMR), charge/orbital ordering, phase separation and so on [6,7,8]. Such properties had potential technical applications in magnetic refrigeration, magnetic sensors, and infrared detectors [9, 10].
The Mn ion in the parent compound (REMnO3) occupies the B position and is all + 3 valence, and the material as a whole exhibits antiferromagnetic insulating properties [11]. When doped with divalent alkaline earth ions, a mixed-valence state of Mn3+ and Mn4+ will appear in the system to hold the electric neutrality. Electrons jump between Mn3+ and Mn4+ via oxygen ions, forming a double-exchange mechanism. Since the strong coupling effect of the electron–phonon, the electrons in the process of jumping are accompanied by the movement of lattice distortion, resulting in the so-called Jon Teller effect [12,13,14,15,16,17]. In general, double-exchange interactions support metal behavior, while the Jahn–Teller effect is in favor of insulator properties [18, 19].
Many factors have a bearing on the properties of LCMO materials, such as the average radius of the A-site cation, the tolerance factor, and oxygen vacancy [20, 21]. However, in perovskite manganese oxides, oxygen vacancy exists as a defect that is easily ignored and quantified by parameter δ. Similar to the La/Ca chemical substitution, changes in oxygen content affect carrier concentration and thus transport and magnetic properties. Besides, the effect of grain boundaries in polycrystalline manganese oxides has been intensively studied, and different synthesis methods and preparation conditions can change the microstructure of ceramics such as uniformity, crystallization, and grain size, thus impacting the electrical properties of the system [22,23,24,25]. The conventional methods for the preparation of calcium-titanite manganese oxides include the conventional solid-state reaction, co-precipitation, reactive milling, spray-drying, sol–gel method, etc. [26, 27]. The co-precipitation method has the advantages of solid-phase and sol–gel methods and has the high chemical activity to accurately control the ratio of raw materials, to prepare the precursors with small particle size and uniform distribution. So it is of great significance to study the preparation of LCMO ceramics using the co-precipitation method to improve their magnetoelectric transport performance.
In the present work, LCMO was synthesized through the co-precipitation method and sintered at various temperatures. The crystallinity, microstructure, and electrical properties of these samples were investigated for all samples. The maximum TCR of the samples obtained by sintering at 1450 °C is close to that of the sol–gel method. Furthermore, by fitting ρ-T, the mechanism of the electrical properties in the metallic and insulating regions of the samples were systematically investigated.
2 Experimental
The series of LCMO ceramic samples were prepared by the co-precipitation. La(NO3)3·nH2O, Ca(NO3)2·4H2O, and Mn(NO3)2·4H2O were used as materials, and dissolved the required amount of these metal salts in deionized water in a stoichiometric ratio. (NH4)2CO3 was also dissolved in deionized water as a precipitant, and the two cups of solution were stirred continuously on a magnetic stirrer until homogeneous clarification. Poured the mixture of La(NO3)2, Ca(NO3)2 and Mn(NO3)2 into a brown dropper and the precipitant (NH4)2CO3 into a transparent dropper, and the reaction was carried out by co-dropping the two solutions slowly and uniformly until complete, then stirred for 30 min. Afterward, the reaction solution was left to aging for 14 h in a dark place. The aging mixture was stirred and the precipitate was evenly dispersed into the solution using an ultrasonic disperser and poured into a funnel for filtration. The filter cake was dried in an oven at 70 °C for 7 h. Then the dried material was ground for the first time using an agate mortar, and the resultant powdered white solids were calcined at 500 °C for 8 h. Finally, the calcined precursor powder was ground again, pressed into circular disks with a hydro press at 18–19 MPa. Subsequently, LCMO polycrystalline samples were sintered under different temperatures of 1350 °C, 1400 °C, 1450 °C, and 1475 °C, for approximately 12 h in a muffle furnace.
X-ray diffraction analysis of LCMO ceramics (measurement range 20°–80°, scanning speed 4°/min) was performed to obtain information on the crystalline properties and structure of the samples. The microstructure and surface morphology of the samples were characterized by scanning electron microscopy. The temperature dependence of resistance (R-T) of the sample was measured using the standard four-probe method.
3 Results and discussion
3.1 Structure and surface morphology
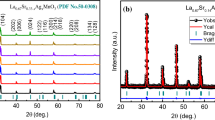
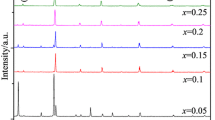
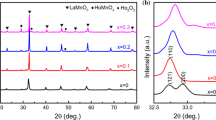
The XRD spectra of the samples prepared at different sintering temperatures are shown in Fig. 1a shows the full diffraction spectrum of LCMO ceramics. The diffraction peaks of all samples were sharp and there are no additional peaks, indicating good crystallization performance, and the structure of all samples was of orthorhombic perovskite. Figure 1b is an enlarged view of the main diffraction peak (121), from which it can be seen that the main peak is shifted to a lower angle and the cell volume should have been increased. According to the Bragg formula and the spacing formula: As the main diffraction peak shifts to a lower angle, the spacing increases, indicating an increase in the cell volume V. To further confirm the altered crystal structure and increased cell volume, XRD spectra were refined for all target samples as shown in Fig. 2, and the resulting fitting parameters are listed in Table 1. From Table 1, it is observed that the cell volume shows a trend of decreasing and then increasing, which can be explained by the absorption and escape of oxygen [28]. In the same air atmosphere (with the same oxygen partial pressure), oxygen absorption plays a key role in the sample when the sintering temperature is increased from 1300 to 1400 °C. At this time, due to the increase of driving force of sintering, the lattice vibration was enhanced, and some oxygen ions diffuse into the lattice. To maintain the electrical neutrality of the system, more Mn3+ is converted into Mn4+, resulting in a decrease in cell volume. However, when the sintering temperature rises more, the sample shows oxygen desorption, and some oxygen will escape from the lattice, showing oxygen vacancy caused by anoxic behavior. Oxygen vacancy is a positively charged hole in the lattice and it has larger than negatively charged oxygen ions [29]. Therefore, desorption of oxygen will increase the unit cell volume. In Fig. 1d–h, it can be seen that the diffraction peaks of the experimental samples are in good agreement with the standard spectra, further confirming the pure-phase calcium-titanite structure of the samples.
The surface morphology of the LCMO at different temperatures (1350 °C (a), 1400 °C (b), 1450 °C (c) and 1475 °C (d)) polycrystalline studied by SEM are given in Fig. 3a–d.
Figure 4a–e shows the grain size of LCMO at different temperatures of 1350 °C (a), 1400 °C (b), 1450 °C (c), and 1475 °C (d). Due to the large grain size of LSMO, to better count the grain size of the material using Nona measurer 1.2 software, then analyze the grain size trends for this series of samples, the SEM image of LSMO at 2000-times magnification was used. It can be seen from the figure that with the increase of sintering temperature, the material becomes denser and the grain size increases. This is mainly because the increase in sintering temperature provides sufficient energy for cell formation and growth, which in turn leads to a decrease in porosity and an increase in crystallinity and density. Meanwhile, with the increase of sintering temperature, the amount of oxygen required increases, resulting‘ in the oxygen deficiency of the samples, which will lead to the increase of grain size [30]. The increase in grain size also leads to a decrease in grain boundaries, which affects the scattering of electrons at the grain boundaries. The grain boundary is highly connected and the reduction in quantity ensures that the material has good electrical properties.
3.2 Temperature dependence of electrical properties (ρ-T)
The resistivity-temperature curves for these samples under different sintering temperatures are shown in Fig. 5. As can be seen in the figure, all samples show similar resistivity trends. As the testing temperature increases, the target is gradually transformed from a ferromagnetic metallic phase at low temperatures to a paramagnetic insulating phase at high temperatures. On the other hand, Tp also showed almost the same change trend as the cell volume. This may be due to the gradual formation of more oxygen vacancies in the sample with the increase of sintering temperature, resulting in the reduction of Mn4+ and the generation of point defects in the structure. Therefore, by changing the bond length and bond angle between manganese ions, the double-exchange effect is weakened, shifting Tp to the low-temperature side. As is well-known, the grain size and the connections between the grains play a important role in electron conduction [31]. With the gradual increase of the sintering temperature, the resistivity of the corresponding sample gradually decreases, from 0.03338 Ω at 1350 °C to 0.01806 Ω at 1475 °C. The reason is, raising the sintering temperature promotes grain growth and amalgamation, reduces porosity, and improves intergrain contact. Meanwhile, based on the double-exchange model, Mn3+ and Mn4+ in LCMO transfer charge through oxygen ions, and the local change of oxygen vacancy concentration will lead to the change of resistivity [32]. Therefore, with the increase of sintering temperature Ta, some crystal cells in LCMO may form oxygen vacancies due to the lack of oxygen, which increases oxygen ion mobility. Thus, the sample exhibits an increase in electrical conductivity.
3.3 Temperature coefficient of resistivity (TCR)
Based on the above discussion, it can be seen that the steepness of the R-T curve transition varies for these samples with the temperature reaching Tp. To explain this phenomenon, the resistive temperature coefficient TCR has been investigated, which can be defined by Eq. (1):
where ρ is the resistivity and T is the temperature.
Figure 6 shows the TCR curve of LCMO samples. TCRmax tended to increase and then decrease at different sintering temperatures. The TCR reached the maximum of 32.3%·K−1 for the sintering temperature of 1450 °C. Furthermore, it is noteworthy that the transition broadening temperature (ΔT) becomes progressively narrower along with increasing sintering temperature. (ΔT) depicts the temperature range during the metal-insulation transition. Resistivity changes concerning grain boundaries, yet (ΔT) is determined by the degree of synchronization within the grain boundaries or between different grains. This can be explained by the homogeneity within the polycrystal, where the more grain boundaries there are, the less homogeneous the sample will be. In the increase of sintering temperature from 1350 °C to 1450 °C, the ratio of Mn4+/Mn3+ in the samples increased, resulting in an enhanced double-exchange interaction. Meanwhile, the grain size increases and the grain boundaries decrease, thus improving the uniformity of the sample and contributing to the enhancement of TCR. However, when the temperature is increased to 1475 °C, the increase of oxygen vacancy, the vacancy states formed by Mn and O in the cell where the vacancy is located destroy the spin polarization, which affects the metallicity of LCMO. In the meantime, the crystallinity of the sintered sample may decrease, which may manifest itself as a decrease in TCR. Which was noted (seen in Fig. 5) that at the sintering temperature of 1500 °C, the samples exhibited no metallic-insulation transition and showed insulating properties. It is confirmed that the sintering temperature of 1500 °C exceeds the melting point of La0.67Ca0.33MnO3 ceramics, not suitable as a conventional sintering temperature. By combining the electrical properties of the sintering temperature of 1450 °C and 1475 °C, 1450 °C was selected as the conventional sintering temperature for La0.67Ca0.33MnO3.
3.4 Conduction mechanism
To better understand the electrical transport mechanism of LCMO, the ρ-T curve of the sample is divided into the metallic phase region (T < Tp) and the insulating phase region (T > Tp). The experimental data in different temperature regions have been combined with the corresponding formula model for the fit analysis.
3.4.1 Low-temperature range
The samples have presented in the metallic phase at low temperatures. To study the influence of the scattering mechanism on the resistivity of the sample, the resistivity data were fitted in the low-temperature phase by Eq. (2) [33, 34]:
where \( \rho _{0} \) is the residual resistivity, mainly due to grain boundary scattering, \({\rho }_{2}\) T2 is the scattering effect between electrons and electrons. \({\rho }_{4.5}\) T4.5 represents the complex scattering interaction to the combination of electron, phonon and magneton. The fitting graphs at the region of T < Tp are shown in Fig. 7a and the relevant parameters obtained are listed in Table 2. As seen in Table 2, the residual resistivity \({\rho }_{0}\) and \({\rho }_{2}\) T2 gradually decreases as the higher the sintering temperature. This is mainly due to the increased grain size of the sample, which reduces the scattering process by decreasing the quantity of grain boundaries, corresponding to the SEM results. The magnitude(seen in Table 2) could be observed that \({\rho }_{0}\) > \({\rho }_{2}\) > \({\rho }_{4.5}\). Thus, it can be demonstrated that an increase in grain size can reduce the grain boundary region, thus decreasing the net grain boundary scattering term as well as the electron-magneton scattering term. This explored that the residual resistivity \({\rho }_{0}\) due to grain boundary scattering in this low-temperature range (T < Tp) is the dominant factor in the electrical transport performance of the sample.
3.4.2 High-temperature range
To investigate the mechanism of ρ-T in the paramagnetic high-temperature region (T > Tp), the ρ-T were fitted through the small-polaron hopping model as Eq. (3):
where \({\rho }_{0s}\) is the resistivity coefficient, \({E}_{a}\) is the small-polaron activation energy and \({k}_{\mathrm{B}}\) is Boltzmann constant. The model fitting result is shown in Fig. 7b. The parameters are presented in Table 3. As seen, with the sintering temperature increasing, the overall activation energy \({E}_{a}\) tends to decrease. Widely known, the interconnectivity between the grains increases with the grain size increasing, which will increase the likelihood that conductive electrons jump to neighboring positions. Also, the sintering drive at high sintering temperatures makes the conduction electrons more prone to transition. This increases the number of carriers excited from the valence band to the conduction band, leading to a decrease in the activation energy Ea, and thus causing a change in the electrical transport properties of the sample.
4 Conclusion
In this paper, the effects of sintering temperature on the electron transport properties of La0.67Ca0.33MnO3 ceramics prepared by co-precipitation were studied. XRD analysis demonstrated that all polycrystalline samples belong to the orthorhombic calcite structure with a space group, with good crystallization properties. SEM images showed that the samples with different sintering temperatures have a higher density and larger grain sizes at higher temperatures, which is the main reason for the decreased resistivity. The highest TCR (TCRmax = 32.3%·K−1) is obtained at a sintering temperature of 1450 °C. The analysis shows that the combined effect of the oxygen vacancy and grain size might be responsible for the observed electrical behavior. The conduction mechanism in the low-temperature region (T < Tp) can use the grain boundary scattering effect of the sample to explain, and the ρ-t curve in the high-temperature region can be fitted by the adiabatic small dipole model.
References
E. Rozenberg, M. Auslender, A.I. Shames, Y.M. Mukovskii, E. Sominski, A. Gedanken, Specific effects of nanometer scale size on magnetic ordering in La1−xCaxMnO3 (x=0.1, 0.3 and 0.6) manganites. J. Non Cryst. Solids 354, 5282 (2008). https://doi.org/10.1016/j.jnoncrysol.2008.05.087
Q. Zhou, M. Dai, R. Wang et al., Enhancement of magnetoresistance in Mn substituted La2/3Sr1/3Mn1−xZrxO3 granular system. Phys. B 371, 120 (2006). https://doi.org/10.1016/j.physb.2005.09.030
S.P. Altintas, A. Amira, C. Terzioglu, Structural characterization and magneto electrical behavior of Sm doped La0.7Ca0.3MnO3 manganites. J. Supercond. Nov. Magn. 26, 1461 (2012). https://doi.org/10.1007/s10948-012-2065-4
J. Ma, Y. Cai, W. Wang et al., Enhancement of temperature coefficient of resistivity in La0.67Ca0.33MnO3 polycrystalline ceramics. Ceram. Int. 40, 4963 (2014). https://doi.org/10.1016/j.ceramint.2013.10.105
S. Vadnala, P. Pal, S. Asthana, Investigation of near room temperature magnetocaloric, magnetoresistance and bolometric properties of Nd0.5La0.2Sr0.3MnO3: Ag2O manganites. J. Mater. Sci. Mater. Electron. 27, 6156 (2016). https://doi.org/10.1007/s10854-016-4543-0
N. Raju, D.R. Sree, S.S.K. Reddy et al., Nanosize effects on the magnetic field induced transitions in La0.67−xEuxCa0.33MnO3 perovskite manganite. J. Magn. Magn. Mater. 368, 308 (2014). https://doi.org/10.1016/j.jmmm.2014.05.029
S. Zhao, X. Yue, X. Liu, Tuning room temperature T p and MR of La1-y(Cay-xSrx )MnO3 polycrystalline ceramics by Sr doping. Ceram Int. 43, 4594 (2017). https://doi.org/10.1016/j.ceramint.2016.12.121
K. Navin, R. Kurchania, The effect of particle size on structural, magnetic and transport properties of La0.7Sr0.3MnO3 nanoparticles. Ceram Int. 44, 4973 (2018). https://doi.org/10.1016/j.ceramint.2017.12.091
D.J. Singh, W.E. Pickett, Lattice effects in ferromagnetic manganite perovskites. J. Appl. Phys. 83, 7354 (1998). https://doi.org/10.1063/1.367678
P. Zhang, T.L. Phan, S.C. Yu, Magnetocaloric Effect in La0.7Cd0.3MnO3, La0.7Ba0.3MnO3, and Nd0.7Sr0.3MnO3. J. Supercond. Nov. Magn. 25, 2727 (2011). https://doi.org/10.1007/s10948-011-1252-z
J.C. Debnath, A.M. Strydom, Transport-entropy correlations in La0.7Ca0.3MnO3 manganite. Phys. B 432, 96 (2014). https://doi.org/10.1016/j.physb.2013.09.045
Y. Zhou, X. Zhu, S. Li, Structure, magnetic, electrical transport and magnetoresistance properties of La0.67Sr0.33Mn1−xFexO3(x=0-0.15) doped manganite coatings. Ceram Int. 43, 3679 (2017). https://doi.org/10.1016/j.ceramint.2016.11.210
T.L. Phan, P.S. Tola, N.T. Dang, J.S. Rhyee, W.H. Shon, T.A. Ho, Tricritical behavior and Griffith phase in La1-xCaxMnO3 under high applied fields. J. Magn. Magn. Mater. 441, 290 (2017). https://doi.org/10.1016/j.jmmm.2017.05.088
P.S. Solanki, R.R. Doshi, A. Ravalia et al., Transport studies on La0.8−xPr0.2SrxMnO3 manganite films. Phys. B 465, 71 (2015). https://doi.org/10.1016/j.physb.2015.02.019
V. Ferrari, J.M. Pruneda, E. Artacho, Density functionals and half-metallicity in La2/3Sr1/3MnO3. Phys. Status Solidi A 203, 1437 (2006). https://doi.org/10.1002/pssa.200566183
G. Jung, V. Markovich, D. Mogilyanski, C. van der Beek, Y.M. Mukovskii, Ferromagnetic and twin domains in LCMO manganites. J. Magn. Magn. Mater. 290–291, 902 (2005). https://doi.org/10.1016/j.jmmm.2004.11.285
F.L. Tang, X. Zhang, Y. Shao, Sr ion distribution and local structure in La1−xSrxMnO3. J. Phys. Condens. Matter 18, 5579 (2006). https://doi.org/10.1088/0953-8984/18/23/026
J. Ma, M. Theingi, H. Zhang, X. Ding, Q. Chen, Structural and electrical characterization of La0.72Ca0.28MnO3 ceramic and thin films. Appl. Surf. Sci. 264, 225 (2013). https://doi.org/10.1016/j.apsusc.2012.09.177
H.Y. Hwang, S.W. Cheong, P.G. Radaelli, M. Marezio, B. Batlogg, Lattice effects on the magnetoresistance in doped LaMnO3. Phys. Rev. Lett. 75, 914 (1995). https://doi.org/10.1103/PhysRevLett.75.914
T. Geng, N. Zhang, Electronic structure of the perovskite oxides La1−xSrxMnO3. Mod. Phys. Lett. 351, 314 (2006). https://doi.org/10.1016/j.physleta.2005.10.095
J. Sakai, N. Ito, S. Imai, Oxygen content of La1−xSrxMnO3−y thin films and its relation to electric-magnetic properties. J. Appl. Phys. (2006). https://doi.org/10.1063/1.2176323
V.P.S. Awana, R. Tripathi, S. Balamurugan, H. Kishan, E. Takayama-Muromachi, Magneto-transport of high TCR (temperature coefficient of resistance) La2/3Ca1/3MnO3: Ag polycrystalline composites. Solid State Commun. 140, 410 (2006). https://doi.org/10.1016/j.ssc.2006.09.021
R. Søndenå, P. Ravindran, S. Stølen, T. Grande, M. Hanfland, Electronic structure and magnetic properties of cubic and hexagonal SrMnO3. Phys. Rev. B (2006). https://doi.org/10.1103/PhysRevB.74.144102
P. Nisha, S.S. Pillai, M.R. Varma, K.G. Suresh, Influence of cobalt on the structural, magnetic and magnetocaloric properties of La0.67Ca0.33MnO3. J. Magn. Magn. Mater 327, 189 (2013). https://doi.org/10.1016/j.jmmm.2012.09.029
G.F. Wang, L.R. Li, Z.R. Zhao, X.Q. Yu, X.F. Zhang, Structural and magnetocaloric effect of Ln0.67Sr0.33MnO3(Ln=La, Pr and Nd) nanoparticles. Ceram. Int 40, 16449 (2014). https://doi.org/10.1016/j.ceramint.2014.07.154
F. Jin, H. Zhang, Q. Chen, Improved Curie temperature and temperature coefficient of resistance (TCR) in La0.7Ca0.3-xSrxMnO3:Ag0.2 composites. J. Alloys Compd 747, 1027 (2018). https://doi.org/10.1016/j.jallcom.2018.03.117
L. Li, H. Zhang, X. Liu et al., Structure and electromagnetic properties of La0.7Ca0.3-xKxMnO3 polycrystalline ceramics. Ceram. Int 45, 10558 (2019). https://doi.org/10.1016/j.ceramint.2019.02.120
X.F. Song, G.J. Lian, G.C. Xiong, Small polaronic transport in oxygen-deficient La0.7Ca0.3MnO3-δthin films. Phys. Rev. B (2005). https://doi.org/10.1103/PhysRevB.71.214427
J. Zhao, C. Liu, J. Li et al., Oxygen vacancy induced electronic structure variation in the La0.2Sr0.8MnO3 thin film. AIP Adv (2019). https://doi.org/10.1063/1.5088738
D.R.S.B.K. Roul, S. Mohanty, A.K. Pradhan, Effect of high temperature sintering schedule for enhanced CMR properties of La0.67Ca0.33MnO3 close to room temperature. Mater. Chem. Phys 67, 267 (2001)
J.S. Park, C.O. Kim, Y.P. Lee et al., Influence of grain size on the electronic and the magnetic properties of La0.7Ca0.3MnO3−δ. J. Appl. Phys 96, 2033 (2004). https://doi.org/10.1063/1.1774264
Y.B. Nian, J. Strozier, N.J. Wu, X. Chen, A. Ignatiev, Evidence for an oxygen diffusion model for the electric pulse induced resistance change effect in transition-metal oxides. Phys Rev Lett 98, 146403 (2007). https://doi.org/10.1103/PhysRevLett.98.146403
G.K. Venkataiah, D.C. Vithal, M. Rao, S.S. Bhat, S.V. Prasad, V. Subramanyam, S.V. Reddy, Effect of sintering temperature on electrical transport properties of La0.67Ca0.33MnO3. Phys. B 357, 370 (2005). https://doi.org/10.1016/j.physb.2004.12.001
S.B. Li, C.B. Wang, H.X. Liu et al., Effect of sintering temperature on structural, magnetic and electrical transport properties of La0.67Ca0.33MnO3 ceramics prepared by Plasma Activated Sintering. Mater. Res. Bull 99, 73 (2018). https://doi.org/10.1016/j.materresbull.2017.10.049
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.11564021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exists in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, L., Li, Y., Yu, P. et al. Exploring the electrical transport properties of La0.67Ca0.33MnO3 at different sintering temperatures. J Mater Sci: Mater Electron 32, 14164–14173 (2021). https://doi.org/10.1007/s10854-021-05943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05943-1