Abstract
As the cathode is a central component of Solid Oxide Fuel Cells (SOFCs), evaluation of cathodes for SOFCs has become a favorite research subject. In the present research, a comprehensive impedance spectroscopy (EIS) analysis is performed to understand the effect of zirconium replacement by cerium on polarization characteristics of La0.7Sr0.3MnO3 (LSM)–Zr0.84Y0.16O1.92 (YSZ8) composite cathode. Three solid oxide fuel cell electrolytes, Ce0.42Zr0.42Y0.16O1.92 (CZY), Ce0.84Y0.16O1.92 (CY) and YSZ8 prepared by solid state reaction, were mixed with appropriate amount of LSM compound to prepare three composite cathodes named LSM–x wt% CZY, LSM–x wt% CY and LSM–x wt% YSZ (x = 30, 50 and 70). The X-ray diffraction analysis has been revealed that LSM is chemically agreeable with all electrolytes, and no undesired reaction happened between them throughout the sintering procedure up to 1350 °C. The thermal expansion coefficients (TECs) for all the prepared electrolyte materials and LSM cathode, show that they are thermomechanically compatible with each other. Results of the electrochemical analysis of symmetrical cells show that the lowest polarization of 0.38 is found by the composition 0.5LSM–0.5YSZ and the highest polarization resistance of 2.72 Ω cm2 is exhibited by the composition 0.3LSM–0.7CZY in the temperature range of 600–850 °C. The composites having YSZ8 and CZY sintered at 1250 °C and CY sintered at 1350 °C showed the maximum electrochemical performance of the cathodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
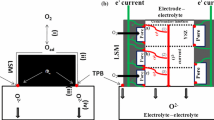
Nowadays Solid Oxide Fuel Cells (SOFCs) are known as an efficient, energy-saving, fuel flexible, and environmentally-friendly power generator device. However, SOFCs are well known and studied but they suffer from some difficulties such as interfacial diffusion, thermal mismatch, and large electrodes over potential [1,2,3,4,5,6,7]. A possible solution for these problems can be lowering the SOFCs working temperature which has been the most important topics of many recent studies concerning SOFCs [8,9,10]. For lowering operating temperature of SOFCs different strategies have been introduced and applied. Using new electrolyte and electrodes materials with high ionic/electronic conductivity and catalytic activity at lowers temperatures, modifying structure–microstructure of SOFC’s layers/interfaces and recently using semiconductor–ionic heterostructure composite electrolytes have been proved to be an effective way for fabricating a low-to-intermediate working temperature SOFCs [11,12,13]. Cerium oxide-based compounds with high ionic conductivity have been introduced as a potential material for using as electrolyte and ionic conductive phase in composite cathode materials [14, 15] and also they have been proved as a semiconducting phase for fabricating semiconductor–ionic heterostructure composite electrolytes [13]. Recent developments have demonstrated a very interesting new trend for designing SOFCs in which semiconductor–ionic membrane are introduced to replace conventional electrolyte for fabricating a new generation of low temperature SOFCs (LTSOFCs) and proton ceramic fuel cells (PCFCs) for low temperature application [16, 17]. Semiconducting characteristics of CeO2-based oxide materials may also improve performance of the composite cathodes as a second phases. In fact the cathode materials should possess appropriate microstructure, high ionic–electronic conductivity and catalytic activity toward oxygen reduction reaction for minimizing cathodic over-potential [5, 18]. Composite cathodes based on an electro-catalytic compound with high electronic conductivity and appropriate amount of an ionic conducting phase with semiconducting characteristics is supposed to possess low polarization overvoltage at relatively low temperature. LSM due to its strong electrochemical activity, electrocatalytic properties and high temperature chemical durability is known as a reliable cathode and it also shows a good thermal compatibility with the conventional electrolyte YSZ which in result reduces the thermal stresses through fabrication and operation of fuel cell device [19, 20]. However, as the LSM material is a purely electronic conductor, it is required to be mixed with an ionic conductor to fabricate a mixed electronic–ionic conductor cathode for extending the cathode reaction from the interface of cathode–electrolyte to all parts of the cathode layer. Furthermore, for the mixed conducting composite cathode materials it would be possible to tailor electronic or ionic conductivity by adjusting the amount of ionic conducting second phase in the prepared composite, for optimizing cell performance [20, 21]. To fabricate composite cathode, especially for the SOFCs in which YSZ or cerium oxide-based materials (e.g., GDC or SDC) is used as the electrolyte, composite cathodes are usually fabricated based on a mixture of the used electrolyte and LSM materials [22, 23]. By now, many investigations have been carried out on such dual phase mixed conducting materials. Moon et al. revealed that the volume fraction of the YSZ phase plays a vital role in the optimization of the cathode microstructure of LSM–YSZ to achieve good stability [24, 25]. The study done by Murray and Barnett shows that the LSM–YSZ50 composite cathode exhibits lower polarization resistance related to pure LSM cathode [26]. Recently, Budiman et al. studied the effect of GDC addition on the electrochemical properties of the LaNi0.6Fe0.4O3−δ–Ce0.9Gd0.1O1.95 composite electrodes [27]. They showed that by increasing GDC phase up to 50 vol%, area specific conductivity increase and leads to enhancement of electrochemical performance of the cathode layer. Based on these outcomes we can conclude that composite cathodes with extended 3D TPB into bulk have superior performance than pure cathodes [26, 28,29,30]. Intuitively, the schematic of the reaction at the composite cathode electrode with extended 3D TPB is shown in Fig. 1, in which the YSZ electrolyte SOFC cathode allows oxygen ions and electrons transportation simultaneously.
In the present work, the performance of LSM material in combination with yttria doped zirconia/ceria (Zr0.84 − xCexY0.16O1.92, x = 0, 0.42 and 0.84) oxide phase is studied comprehensively using electrochemical impedance spectroscopy (EIS) method as a function of the prepared cathode’s composition and microstructures.
2 Experimental procedure
2.1 Sample preparation
As the preliminary materials, commercial 8YSZ (TOSOH, Japan, purity 99.9%), La0.7Sr0.3MnO3 (LSM), yttrium, zirconium and cerium oxide (Inframat® Advanced Materials™ Co., USA, purity 99.9%) powders were used. CY (Ce0.84Y0.16O1.92) and CZY (Ce0.42Zr0.42Y0.16O1.92) electrolytes were prepared by solid state synthesizing method, more details about the procedure is reported in our previous work [11]. CZY, CY and YSZ were mixed with the desired amount of LSM, individually, in ethanol using the attrition mill with ZrO2 milling media for 4 h. After that the powder mixtures were heated at 90 °C for 2 h and subsequently sieving using a 60-mesh sieve, were used for fabricating symmetrical cells to evaluate the composite cathode materials electrochemical performances. To fabricate symmetrical cells, first at all, the electrolyte pellets (thickness = 1 mm, diameter = 10 mm) were prepared by pressing 0.35 g of YSZ powders under 100 MPa pressure and sintering at 1450 °C for 2 h. Then, the composite cathodes inks painted on both sides of the sintered YSZ electrolytes, while painting inks were prepared by dispersing the composite-cathode powders into terpineol as organic solvent including 3 wt% ethyl cellulose as binder, in an agate mortar for 30 min. Different LSM–CZY, LSM–CY and LSM–YSZ cathode layers were prepared with the equal thickness of 25 ± 5 µm. Then the fabricated cells with composite cathodes (0.5LSM–0.5CZY, 0.5LSM–0.5CY and 0.5LSM–0.5YSZ) were sintered at different temperatures (1050–1350 °C) for 3 h in air to study the effect of sintering temperature on the microstructure and the electrochemical activity.
2.2 Characterization
Phase purities of the synthesized electrolytes and chemical compatibility of 0.5LSM–0.5YSZ, CY and CZY cathode–electrolyte mixtures were examined by analyzing X-ray diffractometer (XRD) with Cu Kα radiation (λ = 1.54060 Ǻ) as the source. Thermal expansion coefficients (TECs) of the LSM, 0.5LSM–0.5CZY, 0.5LSM–0.5CY and 0.5LSM–0.5YSZ cathode samples that sintered at 1350 °C for 5 h, with a density more than 95% of theoretical density, were measured in the temperature between 30 and 800 °C in static air at a heating rate of 5 °C/min using a horizontal dilatometer (LINSEIS L75).
The electrochemical impedance spectroscopy techniques (EIS) were applied on the symmetrical cells to investigate the electrochemical behavior of the cells with different composite cathodes as function of temperature in the range of 600 and 850 °C in the air using a Solartron 1260–1287 impedance. The applied bias voltage was 40 mV with a frequency range from 1 MHz to 0.1 Hz. To prepare the samples for EIS test, Ag paste as electrode counter was painted on the both sides of cathode layers then sintered at 800 °C for 1 h. The cells were placed and forced with a soft spring between two alumina discs, which were covered with Ag mesh as current collector, while they were in an alumina supporting tube, inserted in a tubular furnace. The cell was connected to the electrochemical instruments through silver wires, which were shielded to reduce noise. After carrying out the electrochemical investigation, scanning electron microscopy (SEM) was applied to analyze the microstructures of cathode and electrolyte at the interface and also cathode layer, thickness uniformity and other relevant morphological features.
3 Results and discussion
3.1 Powder characteristics
To identify the formation of probable secondary phases, X-ray patterns of the synthesized CY, CZY and commercial YSZ, LSM powders are compared with 0.5LSM–0.5CZY, 0.5LSM–0.5CY and 0.5LSM–0.5YSZ composites sintered at 1350 °C for 3 h with the heating rate of 3°/min. The results are shown in Fig. 2. As can be seen, the diffraction peaks of CZY, CY and YSZ show a pure phase without impurities peaks that can be matched with the cubic structure, whereas the diffraction peaks of LSM well-adjusted to a perovskite structure. In the diffraction patterns of composites, only the initial phases can be clearly identified without any other secondary phases or peak shifts, verifying that the two phases co-existed in the composite and no objectionable reaction happened in the firing process at least up to 1350 °C for 3 h. In the other words, as the kinetic of reaction between LSM and YSZ is slow; therefore, sintering time of 3 h at 1350 °C would not result in creation of second phase or even significant dissolution of Mn in YSZ, because both processes are controlled by diffusion which is not enough in 3 h. Chih-Chung et al. reported that cofiring of LSM–YSZ at 1400 °C for 1 h do not result in creation of second phase [31]. They showed that creation of second phases starts after 10 h cofiring at 1400 °C. Therefore, it can be concluded that not only LSM has good chemical compatibility with the YSZ phase [32] but also it is chemically compatible with CY and CZY phases, and therefore, they can be used together with LSM material for making cathode composite in solid oxide fuel cell devices.
3.2 Thermal expansion behavior
The thermal in accordance between electrolyte and cathode materials is an important factor for choosing effective cathode compositions. To avoid delamination, internal stresses, and cracks which intensely influence the long-term stability of SOFCs, the cathode materials must own thermal expansion coefficient that matches the other parts in SOFCs, specifically the electrolyte membrane [32,33,34,35]. Figure 3 exhibits the curves of thermal expansion for CZY, CY, YSZ and LSM compounds. It has been observed that thermal expansion of all samples has a linear relationship with the temperature. The obtained average values of TECs between 25 and 800 °C, are also presented in Fig. 3, which indicates that the TECs values of all electrolyte samples were close to the TEC value of LSM material, suggesting that their addition to LSM would not alter the TEC of the composite cathode [35, 36].
3.3 Morphology analysis
To investigate the influence of sintering temperature on the microstructure of 0.5LSM–0.5CZY, 0.5LSM–0.5CY and 0.5LSM–0.5YSZ cathode composites, the cathode samples were sintered at various temperatures ranging between 1050 and 1350 °C for 3 h. Figure 4 exhibits the SEM cross-sectional images of 0.5LSM–0.5CY composite electrode sintered at 1050, 1150, 1250, 1350 °C. The sintering at 1350 °C was considered to get the biggest grain size having the smallest amount of remained porosities in the cathode layer. This condition is useful to evaluate the behavior of electrode as a function of its morphological characteristics [32]. A careful observation of the cathode morphologies proved a well-developed interaction among the particles and a fine structure is obtained. However, for the sample sintered at 1050 °C, the remind porosity is plentiful and no good connections were created between the particles (Fig. 4a). The increase in sintering temperature to 1250 °C, led to the formation of a well-connected porous network (Fig. 4c), which helps to improve the catalytic reaction of the oxygen reduction. But at the sintering temperature over 1250 °C, the particle size was increased so much and could not make a well-bonded porous network, as it is shown in Fig. 4d, which can cause a significant decrease in the electrode’s electrical conductivity [6]. Figure 4e demonstrates the cross-sectional view of the electrolyte–electrode layers, showing a well-developed continues contact between these two layers and revealed that the cathode layer is about 27 μm thick. The same trend was observed for other composite cathodes, and therefore, their SEM images are not presented.
3.4 Effect of LSM–CZY, LSM–CY, LSM–YSZ cathodes microstructures on electrochemical performance of the symmetrical cell
In a recently published paper by the present authors [20], the electrical conductivity of the cathodes having these composition, i.e., LSM–CZY, LSM–CY and LSM–YSZ, are evaluated and presented. The superior electrical conductivity of CY and CZY, similar to YSZ, encouraged us to study if these solid solutions can also be considered as a candidate to be used as an ion-conducting compound in preparation of composite cathodes for solid oxide fuel cells application. In the current work, electrochemical impedance spectroscopy (EIS) was performed on the symmetrical cells prepared using LSM–CZY, LSM–CY and LSM–YSZ composite cathodes on YSZ electrolytes to investigate their electrochemical performance and to evaluate interfacial polarization resistances for better understanding the electrode processes. All cathodes prepared in this study had a homogeneous thickness of around 25–30 μm. In the first step, for finding the appropriate sintering temperature for the used composites, the influence of various sintering temperatures on the electrochemical behavior of the composite cathodes was investigated. So, in this case, for the electrodes sintered at various temperatures in the range of 1050–1350 °C, electrochemical impedance (EIS) measurements were carried out at different temperatures, i.e., in the range of 600–850 °C in air, and the results are shown in Figs. 5, 6 and 7. It is necessary to mention that for better comparison between different spectra, in all Nyquist plots ohmic resistance of the cells was eliminated. Results showed that the microstructural of the used electrodes have a great influence on the cathodic action because of the notable shift in the solid/gas surface area and the three-phase boundary (3-PB) length [32]. The impedance spectra show a typical shape, where at the high and low frequency, the gap between the two intercepts on the real axis of the electrochemical impedance spectra (EIS) is related to the polarization resistance (Rp) of the applied cathode, which has been used as the main parameter for comparing the cathodes performances [37]. It is observed that the Rp significantly decreases by increasing the operating temperature, indicating the temperature has a significant and direct effect on the cathode activity. Arrhenius plots of ln (Rp) vs. 1000/T for all the cathode composites sintered at various temperatures are presented in Fig. 8. It can be witnessed that the resistance polarization (Rp) of the 0.5LSM–0.5CY and 0.5LSM–0.5CZY composite cathodes decreased by increasing sintering temperature, even as high as 1350 °C. For the 0.5LSM–0.5YSZ composite cathode, a different trend was observed so that the polarization resistance decreased by increasing sintering temperature up to 1250 °C and then increased by more increasing in sintering temperature. The cathode materials having huge surface area, adequate grain size, and enough porosity and good compatibility with the electrolyte layers exhibit high performance. It can be noticed that, at the lower sintering temperature (e.g., 1050 °C), the connections among the agglomerated particles of the cathode material are poor and the electrolyte and cathode are not well adhered to each other, which strongly resist the electron transfer and bulk-surface diffusion of oxygen ions through the porous cathode material. By increasing the sintering temperature (up to, e.g., 1250 and 1350 °C) bonding between the cathode particles can be improved; however, the active sites for oxygen reduction reaction and electrode’s porosity are decreased because of the growth of the cathode particles and decreasing the amount of remained porosities, that may result in bigger Rp [38, 39]. Therefore, an optimum sintering temperature can be defined at which the Rp is minimum. This temperature was determined around 1250 °C for 0.5LSM–0.5YSZ composite cathode. For the 0.5LSM–0.5CZY and 0.5LSM–0.5CY composite cathodes the minimum values of Rp were found to be 1 Ω cm2 and 0.6 Ω cm2, respectively, for the sintering temperature of 1350 °C.
Regarding the presented data in Fig. 8, it can be noted that by replacing zirconium with cerium in YSZ material, the optimum sintering temperature for achieving minimum Rp value increases. Considering the impedance in the temperature range of 600–850 °C of symmetrical cell with 0.5LSM–0.5CZY composite cathode sintered at 1250 and 1350 °C, it can be noted that the Rp values were similar at high temperature, while at intermediate and low temperature, the Rp value of the cathode sample sintered at 1350 °C was found to be lower. The summary of the polarization resistance for the composite cathodes including LSM–50 wt% YSZ, LSM–50 wt% CZY and LSM–50 wt% CY, sintered at different temperature are summarized in Table 1.
Furthermore, as it can be seen in Fig. 8, there is a linear relationship between 1000/T and ln (Rp) for all the prepared composite cathodes, which proved that the kinetics of the cathodic process is under activation control [40]. The activation energy for the cathodic reaction in all three different composite cathodes prepared in this study was determined in the range of 110–150 kJ mol−1, nearly the same as what reported by other researchers for LSM:YSZ composite cathodes [41]. However, the lowest activation energy (110 kJ mol−1) was calculated for the LSM–50 wt% CY composite cathode sintered at 1350 °C, indicating that it has a fast-catalytic activity for ORR (oxygen reduction reaction) and oxygen permeability. The highest activation energy (149.63 kJ mol−1) corresponded to the 0.5LSM–0.5CZY composite cathode sintered at 1250 °C, which shows the LSM–CZY interlayer need to be further optimized to improve oxygen reduction reaction activity. The activation energy for the other composite cathodes is presented in Tables 2 and 3. As it can be seen, sintering temperature has different effect on the activation energy of the cathode polarization of different composite cathodes including the same amount of LSM and different type of ionic conductors. In the other words, it is expected to have lower activation energy for the composite cathodes with high electronic/ionic conductivity. The LSM–CY composites, with the highest electrical conductivity among the fabricated composites [20], show the lowest activation energy, implying that the addition of CY further increases oxygen reduction reaction activity.
Considering the data presented in Table 2, it can be seen that for the LSM–YSZ composite by increasing sintering temperature more than 1150 °C, activation energy increase that may be as a result of very small dissolution of Mn in YSZ phase, although it cannot be recognized in XRD patterns [41].
3.5 Comparing the electrochemical performance of LSM–CZY, LSM–CY and LSM–YSZ composites
The LSM–x wt% CZY, LSM–x wt% CY and LSM–x wt% YSZ (x = 30, 50 and 70) composites-cathode’s impedance spectra obtained at 850 °C, are presented in Fig. 9. The calculated polarization resistance, Rp, at 850 °C for these composite cathodes, sintered at 1250 °C, are tabulated in Table 3. As it can be seen, polarization resistance of the LSM–x wt% CZY and LSM–x wt% YSZ composites were decreased by increasing YSZ and CZY content up to 50 wt% and then increased, so that the 0.5LSM–0.5YSZ and 0.5LSM–0.5CZY have the least polarization resistance. In the case of the LSM–x wt% CY composites, 0.3LSM–0.7CY has the lowest polarization resistance and very close to Rp of 0.5LSM–0.5CY composite. Since the ionic conductivities of YSZ, CY and CZY are high, thus it offers more electrochemically active sites from the electrolyte/cathode interface to the whole cathode layer thickness, in result, the cathode performance is improved and the reduced Rp is expected by their addition to LSM material. On the other hand, as the amount of YSZ and CZY has increased up to 50 wt%, due to the weak electrical conductivity of YSZ and CZY, the electrical conductivity of the LSM–x wt% YSZ and LSM–x wt% CZY composites decreases which resists the charge transport, current collection and electrical conduction [3, 6, 20]. The same trend is also expected when YSZ or CZY has been used more than 50 wt% in the composites. Also, the presence of YSZ and CZY on the LSM backbone hinders the connectivity of LSM particles, creating small active paths for electronic transport at electrode side and causing a rise in Rp. Comparing the electrochemical performance of two composites cathode including YSZ and CZY, it can be concluded that an increase in polarization resistance due to use of more than 50 wt% of ionic conductive phase were more pronounced in LSM–CZY composites [42, 43].
For the LSM–x wt% CY composites, as can be seen in Fig. 9, the trend is a little different from what reported for the two other composite electrodes. It is noticeable that 0.3LSM–0.7CY composite has lower polarization resistance than other composites including CY as the ionic conductor phase. This may be due to the greater conductivity of LSM–x wt% CY with respect to the composites including YSZ or CZY ionic conductor phases [20]. Even though the triple phase boundaries (TPBs) are extended to the 3-D bulk of the composite electrodes, the electrical conductivity is still high. However, as the pure oxygen ion conductivity of YSZ electrolyte is higher than that of CY; therefore, lower polarization resistance for the composites including YSZ is expected [38].
Figure 10 shows the electrochemical performance of different prepared symmetrical cells with all LSM–CZY, LSM–CY and LSM–YSZ composite cathodes and with different amount of LSM, at 850 °C. As can be seen, the variance in electrochemical performance of the composites is not persistent and effects by change in LSM phase. In Fig. 11, the behavior of Rp as a function of temperature for these cathodes is shown. It can be seen, in all samples with the same amount of LSM, the LSM–YSZ composites have the least polarization resistance and the difference in polarization resistance between LSM–YSZ and the two other composites is big; meanwhile, the used ionic conductive phase is less (30 wt%). Using the 50 wt% amount of the ionic conductive phase, the difference is lesser. For the composites including the highest amount of electrolyte phase (70 wt%), polarization resistance of the LSM–CY and LSM–YSZ composites are almost equal at various temperatures and they are lower than the 0.3LSM–0.7CZY composite. It can be seen in Table 2, in all cathode composites with the same amount of LSM, LSM–CY composite cathodes have the lowest activation energy especially 0.3LSM–0.7CY composite with 118 kJ mol−1. The decreased activation energy with CY suggests that the introduction of high percent of CY powder makes the oxygen reduction reaction easier and faster.
4 Conclusion
Electrochemical performance of composite cathodes of LSM–x wt% yttria doped zirconia/ceria [Zr0.84 − xCexY0.16O0.96 (x = 0, 0.42, 0.84)] has been reported as a function of composition and microstructure for cathode application in SOFCs. XRD results confirmed a single cubic phase for all the zirconium-based fabricated electrolytes and good chemical compatibility between the synthesized phases and LSM material. SEM images demonstrated a good correlation between the applied sintering temperature and microstructural change, while it also showed a well-developed interface between cathode and electrolyte layer. An analysis of the thermal expansion coefficient of the synthesized and used ionic conductive materials showed that TEC value of all the electrolytes used in this study was not significantly different from the TEC value for LSM. Based on the electrochemical analysis carried out on symmetrical cells fabricated using YSZ8 electrolyte and composite-cathode materials prepared in this study, it was shown that in the temperature range of 600–850 °C, the polarization resistance of LSM–x wt% YSZ is the minimum and for the LSM–x wt% CZY the polarization resistance is the maximum for all the composition. The effect of sintering temperature on the electrochemical activity for all three 0.5LSM–0.5YSZ and 0.5LSM–0.5CZY composite cathodes, at various temperatures was investigated. It is witnessed that the optimum sintering temperature at which the lowest polarization resistance is recorded to be 1250 °C. Furthermore, it was shown the LSM–CY composite cathodes have the lowest activation energy between all the fabricated composites and the order of its polarization resistance was LSM–YSZ < LSM–CY < LSM–CZY. For the composites having 70 wt% of ionic conducting phase, CY phase showed more efficient performance in decreasing polarization resistance.
References
J. Nielsen, T. Jacobsen, M. Wandel, Impedance of porous IT-SOFC LSCF: CGO composite cathodes. Electrochim. Acta 56, 7963–7974 (2011). https://doi.org/10.1016/j.electacta.2011.05.042
C. Zhang, K. Huang, A new composite cathode for intermediate temperature solid oxide fuel cells with zirconia-based electrolytes. J. Power Sources 342, 419–426 (2017). https://doi.org/10.1016/j.jpowsour.2016.12.084
C. Kim, J. Kim, J. Shin, G. Kim, Effects of composite cathode on electrochemical and redox properties for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 39, 20812–20818 (2014). https://doi.org/10.1016/j.ijhydene.2014.07.007
H.J. Ko, J.-h Myung, J.-H. Lee, S.-H. Hyun, J.S. Chung, Synthesis and evaluation of (La0.6Sr0.4)(Co0.2Fe0.8)O3(LSCF)–Y0.08Zr0.92O1.96(YSZ)–Gd0.1Ce0.9O2−δ(GDC) dual composite SOFC cathodes for high performance and durability. Int. J. Hydrog. Energy 37, 17209–17216 (2012). https://doi.org/10.1016/j.ijhydene.2012.08.099
H.J. Ko, J.-H. Myung, S.-H. Hyun, J.S. Chung, Synthesis of LSM–YSZ–GDC dual composite SOFC cathodes for high-performance power-generation systems. J. Appl. Electrochem. 42, 209–215 (2012). https://doi.org/10.1007/s10800-012-0390-8
J. Zhou, Y. Chen, G. Chen, K. Wu, Y. Cheng, Evaluation of LaxSr2− xFeO4 layered perovskite as potential electrode materials for symmetrical solid oxide fuel cells. J. Alloy. Compd. 647, 778–783 (2015). https://doi.org/10.1016/j.jallcom.2015.05.261
X. Lu, Y. Yang, Y. Ding, Y. Chen, Q. Gu, D. Tian et al., Mo-doped Pr0.6Sr0.4Fe0.8Ni0.2O3-δ as potential electrodes for intermediate-temperature symmetrical solid oxide fuel cells. Electrochim. Acta 227, 33–40 (2017). https://doi.org/10.1016/j.electacta.2016.12.170
R. Raza, B. Zhu, A. Rafique, M.R. Naqvi, P. Lund, Functional ceria-based nanocomposites for advanced low-temperature (300–600°C) solid oxide fuel cell: a comprehensive review. Mater. Today Energy 15, 100373 (2020). https://doi.org/10.1016/j.mtener.2019.100373
L. Fan, B. Zhu, P.-C. Su, C. He, Nanomaterials and technologies for low temperature solid oxide fuel cells: recent advances, challenges and opportunities. Nano Energy 45, 148–176 (2018). https://doi.org/10.1016/j.nanoen.2017.12.044
L. Fan, C. Wang, B. Zhu, Low temperature ceramic fuel cells using all nano composite materials. Nano Energy 1, 631–639 (2012). https://doi.org/10.1016/j.nanoen.2012.04.004
R. Raza, Q. Liu, J. Nisar, X. Wang, Y. Ma, B. Zhu, ZnO/NiO nanocomposite electrodes for low-temperature solid oxide fuel cells. Electrochem. Commun. 13, 917–920 (2011). https://doi.org/10.1016/j.elecom.2011.05.032
L. Fan, B. Zhu, M. Chen, C. Wang, R. Raza, H. Qin et al., High performance transition metal oxide composite cathode for low temperature solid oxide fuel cells. J. Power Sources 203, 65–71 (2012). https://doi.org/10.1016/j.jpowsour.2011.12.017
N. Mushtaq, C. Xia, W. Dong, B. Wang, R. Raza, A. Ali et al., Tuning the energy band structure at interfaces of the SrFe0.75Ti0.25O3−δ–Sm0.25Ce0.75O2−δ heterostructure for fast ionic transport. ACS Appl. Mater. Interfaces 11, 38737–38745 (2019). https://doi.org/10.1021/acsami.9b13044
G. Chen, W. Sun, Y. Luo, Y. He, X. Zhang, B. Zhu et al., Advanced fuel cell based on new nanocrystalline structure Gd0.1Ce0.9O2 electrolyte. ACS Appl. Mater. Interfaces 11, 10642–10650 (2019). https://doi.org/10.1021/acsami.8b20454
L. Li, B. Zhu, J. Zhang, C. Yan, Y. Wu, Electrical properties of nanocube CeO2 in advanced solid oxide fuel cells. Int. J. Hydrog. Energy 43, 12909–12916 (2018). https://doi.org/10.1016/j.ijhydene.2018.05.120
M.A.K.Y. Shah, N. Mushtaq, S. Rauf, C. Xia, B. Zhu, The semiconductor SrFe0.2Ti0.8O3-δ-ZnO heterostructure electrolyte fuel cells. Int. J. Hydrog. Energy 44, 30319–30327 (2019). https://doi.org/10.1016/j.ijhydene.2019.09.145
G. Chen, B. Zhu, H. Deng, Y. Luo, W. Sun, H. Liu et al., Advanced fuel cell based on perovskite La–SrTiO3 semiconductor as the electrolyte with superoxide-ion conduction. ACS Appl. Mater. Interfaces 10, 33179–33186 (2018). https://doi.org/10.1021/acsami.8b10087
N. Hildenbrand, B.A. Boukamp, P. Nammensma, D.H. Blank, Improved cathode/electrolyte interface of SOFC. Solid State Ion. 192, 12–15 (2011). https://doi.org/10.1016/j.ssi.2010.01.028
S.P. Jiang, L. Zhang, Y. Zhang, Lanthanum strontium manganese chromite cathode and anode synthesized by gel-casting for solid oxide fuel cells. J. Mater. Chem. 17, 2627–2635 (2007). https://doi.org/10.1039/B701339F
S. Paydar, M. Shariat, S. Javadpour, Investigation on electrical conductivity of LSM/YSZ8, LSM/Ce0.84Y0.16O0.96 and LSM/Ce0.42Zr0.42Y0.16O0.96 composite-cathodes of SOFCs. Int. J. Hydrog. Energy 41, 23145–23155 (2016). https://doi.org/10.1016/j.ijhydene.2016.10.092
H. Shirani-Faradonbeh, M.H. Paydar, Electrical behavior of the Ruddlesden-Popper phase,(Nd0.9La0.1)2Ni0.75Cu0.25O4(NLNC) and NLNC-xwt% Sm0.2Ce0.8O1.9 (SDC)(x= 10, 30 and 50), as intermediate-temperature solid oxide fuel cells cathode. Ceram. Int. 44, 1971–1977 (2018). https://doi.org/10.1016/j.ceramint.2017.10.140
K. Chen, Z. Lü, X. Chen, N. Ai, X. Huang, X. Du et al., Development of LSM-based cathodes for solid oxide fuel cells based on YSZ films. J. Power Sources 172, 742–748 (2007). https://doi.org/10.1016/j.jpowsour.2007.05.035
S. Jiang, W. Wang, Novel structured mixed ionic and electronic conducting cathodes of solid oxide fuel cells. Solid State Ion. 176, 1351–1357 (2005). https://doi.org/10.1016/j.ssi.2005.03.011
H.S. Song, S. Lee, D. Lee, H. Kim, S.H. Hyun, J. Kim et al., Functionally-graded composite-cathodes for durable and high performance solid oxide fuel cells. J. Power Sources 195, 2628–2632 (2010). https://doi.org/10.1016/j.jpowsour.2009.11.067
H.S. Song, S. Lee, S.H. Hyun, J. Kim, J. Moon, Compositional influence of LSM-YSZ composite-cathodes on improved performance and durability of solid oxide fuel cells. J. Power Sources 187, 25–31 (2009). https://doi.org/10.1016/j.jpowsour.2008.10.092
E.P. Murray, S.A. Barnett, Improved performance in (La, Sr) MnO3 and (La, Sr)(Co, Fe)O3 cathodes by the addition of a Gd-doped ceria second phase. ECS Proc. Vol. 1999, 369–378 (1999). https://doi.org/10.1149/199919.0369PV
R. Budiman, S. Hashimoto, Y. Fujimaki, T. Nakamura, K. Yashiro, K. Amezawa et al., Evaluation of electrochemical properties of LaNi0.6Fe0.4O3−δ-Ce0. 9Gd0.1O1.95 composite as air electrode for SOFC. Solid State Ion. 332, 70–76 (2019). https://doi.org/10.1016/j.ssi.2018.12.023
E.P. Murray, S.A. Barnett, (La, Sr)MnO3–(Ce, Gd)O2–x composite-cathodes for solid oxide fuel cells. Solid State Ion. 143, 265–273 (2001). https://doi.org/10.1016/S0167-2738(01)00871-2
S. Wang, Y. Jiang, Y. Zhang, J. Yan, W. Li, Promoting effect of YSZ on the electrochemical performance of YSZ+ LSM composite electrodes. Solid State Ion. 113, 291–303 (1998). https://doi.org/10.1016/S0167-2738(98)00379-8
J. Piao, K. Sun, N. Zhang, S. Xu, A study of process parameters of LSM and LSM–YSZ composite cathode films prepared by screen-printing. J. Power Sources 175, 288–295 (2008). https://doi.org/10.1016/j.jpowsour.2007.09.078
C.C.T. Yang, W.C.J. Wei, Reaction kinetics and mechanisms between La0.65Sr0.3MnO3 and 8 mol% yttria-stabilized zirconia. J. Am. Ceram. Soc. 87(6), 1110–1116 (2004). https://doi.org/10.1111/j.1551-2916.2004.01110.x
M. Carpanese, D. Clematis, A. Bertei, A. Giuliano, A. Sanson, E. Mercadelli et al., Understanding the electrochemical behaviour of LSM-based SOFC cathodes. Part I—experimental and electrochemical. Solid State Ion. 301, 106–115 (2017). https://doi.org/10.1016/j.ssi.2017.01.007
L. Zhang, M. Liu, J. Huang, Z. Song, Y. Fu, Y. Chang et al., Improved thermal expansion and electrochemical performances of Ba0.6Sr0.4Co0.9Nb0.1O3−δ–Gd0.1Ce0.9O1.95 composite-cathodes for IT-SOFCs. Int. J. Hydrog. Energy 39, 7979 (2014). https://doi.org/10.1016/j.ijhydene.2014.03.055
L.-N. Xia, J. You, Z.-P. He, X.-W. Huang, Y. Yu, Performances of nickel-doped SmBaCo2O5+δ–Sm0.2Ce0.8O1.9 composite-cathodes for IT-SOFC. Int. J. Hydrog. Energy 41, 1176–1186 (2016). https://doi.org/10.1016/j.ijhydene.2015.10.027
S.U. Rehman, R.-H. Song, J.-W. Lee, T.-H. Lim, S.-J. Park, S.-B. Lee, Effect of GDC addition method on the properties of LSM–YSZ composite cathode support for solid oxide fuel cells. Ceram. Int. 42, 11772–11779 (2016). https://doi.org/10.1016/j.ceramint.2016.04.098
Y. Shirai, S.-i Hashimoto, K. Sato, K. Yashiro, K. Amezawa, J. Mizusaki et al., Crystal structure and thermal expansion behavior of oxygen stoichiometric lanthanum strontium manganite at high temperature. Solid State Ion. 256, 83–88 (2014). https://doi.org/10.1016/j.ssi.2013.12.042
H. Shirani-Faradonbeh, M. Paydar, S. Paydar, I. Gholaminezhad, R. Bazargan-Lari, Synthesis and electrochemical studies of novel cobalt free (Nd0.9La0.1)1.6Sr0.4Ni0.75Cu0.25O3.8 (NLSNC4) cathode material for IT-SOFCs. Fuel Cells. 19(5), 578–586 (2019). https://doi.org/10.1002/fuce.201900065
X. Zhu, A. Lusi, C. Zhu, Y. Wang, J. Jin, Performance evaluation of Ca3Co4O9-δ cathode on Sm0.075Nd0.075Ce0.85O2-δ electrolyte for solid oxide fuel cells. J. Alloys Compd. 694, 877–883 (2017). https://doi.org/10.1016/j.jallcom.2016.09.297
A.J. Jacobson, Materials for solid oxide fuel cells. Chem. Mater. 22, 660–674 (2009). https://doi.org/10.1021/cm902640j
A. Barbucci, R. Bozzo, G. Cerisola, P. Costamagna, Characterisation of composite SOFC cathodes using electrochemical impedance spectroscopy. Analysis of Pt/YSZ and LSM/YSZ electrodes. Electrochim. Acta 47, 2183–2188 (2002). https://doi.org/10.1016/S0013-4686(02)00095-6
J. Nielsen, J. Hjelm, Impedance of SOFC electrodes: a review and a comprehensive case study on the impedance of LSM:YSZ cathodes. Electrochim. Acta 115, 31–35 (2014). https://doi.org/10.1016/j.electacta.2013.10.053
J. Kim, W.-y Seo, J. Shin, M. Liu, G. Kim, Composite-cathodes composed of NdBa0.5Sr0.5 Co2O5+δ and Ce0.9Gd0.1O1.95 for intermediate-temperature solid oxide fuel cells. J. Mater. Chem. A 1, 515–519 (2013). https://doi.org/10.1039/C2TA00025C
Z. Gao, X. Ding, D. Ding, L. Ding, S. Zhang, G. Yuan, Infiltrated Pr2NiO4 as promising bi-electrode for symmetrical solid oxide fuel cells. Int. J. Hydrog. Energy 43, 8953–8961 (2018). https://doi.org/10.1016/j.ijhydene.2018.03.164
Acknowledgements
The authors gratefully acknowledge Shiraz University for providing financial support during the research work. The authors are thankful to Mrs. Kaveh for recording the SEM images.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paydar, S., Gholaminezad, I., Shirani-Faradonbeh, H. et al. Evaluating the cathodic polarization of La0.7Sr0.3MnO3–Zr0.84−xCexY0.16O1.92 (x = 0, 0.42, 0.84) composites for SOFCs. J Mater Sci: Mater Electron 32, 11129–11144 (2021). https://doi.org/10.1007/s10854-021-05779-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05779-9