Abstract
The influences of different doping contents of additive B2O3 on the sintering behavior, microstructure and microwave properties of Ba(Mg1/3Nb2/3)O3 (BMN) microwave ceramics were studied. The main phase in the sintered ceramics was crystallized as Ba(Mg1/3Nb2/3)O3 (BMN). Ba2B2O5 as the secondary phase was observed with x ≥ 10. Scanning electron microscopy (SEM) analysis revealed that the photograph of optimally nonstoichiometric ceramics sintered at 900 °C for 5 h displayed a dense microstructure. The dielectric properties and microstructures of BMN ceramics were very sensitive to the nonstoichiometry. The addition of B2O3 had a positive influence on the Q × f value, for example the sample with x = 7 showed the highest Q × f value of 89,800 GHz. Finally, excellent microwave dielectric properties (εr = 31.6, Q × f = 89,800 GHz and τf = 3.6 ppm/°C) were achieved for the sample with x = 7 sintered at 900 °C for 5 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the production of miniaturized devices, low densification sintering temperature is very important for ceramic multilayer structures, thus they can be co-fired with low-loss conductors such as silver or copper. Low temperature co-fired ceramic (LTCC) technology has remarkable advantages over the previous packaging technologies in packaging applications with high density, high speed, good heat dissipation and sealing [1, 2]. In recent years, some ceramic-glass compositions [3,4,5] have been used in microwave devices, such as low dielectric constant materials using alumina and suitable glass combinations, and high dielectric constant materials with low dielectric loss in the range of 20-100. As microwave filters, complex perovskite ceramics Ba(A1/3B2/3)O3 (A = Mg, Zn; B = Ta, Nb) have displayed notable properties for the microwave ceramics. Particularly, Ba(Mg1/3Nb2/3)O3 (BMN) ceramic have broad application prospects in electronic systems with preferable dielectric properties of εr = 32, Q × f = 56000 GHz, τf = 33 ppm/°C [6,7,8]. As we know, the main problem of the BMN sintering is that the densification sintering temperature is approximately 1550 °C, and it takes a long time for annealing to obtain ordered BMN phase [6]. In addition, although BMN ceramic possesses superior Q × f value (56000 GHz [6]), its τf value (33 ppm/°C [6]) is unacceptably high, which needs to be adjusted according to practical application. In fact, the higher the sintering temperature is, the higher the cost is. So, it is necessary to reduce the densification sintering temperature to save the manufacturing cost. In order to reduce the sintering temperature and improve the microwave dielectric properties, a lot of researches have been carried out. Tian [9] added 3 mol.% BaWO4 to BMN ceramics and lowered the sintering temperature to 1500 °C, obtaining εr = 31, Q × f = 82,300 GHz and τf = 32 ppm/°C. Shan [10] used 0.25 wt% V2O5 to reduce the sintering temperature of the ceramics system to 1350 °C, achieving superior microwave dielectric performance (εr = 31.7, Q × f = 42,100 GHz and τf = 22.7 ppm/°C). Sun [11] reported that Ba([Mg1-xCox]1/3Nb2/3)O (x = 0.8) ceramics could be well sintered at 1400 °C, and good microwave dielectric performance of εr = 31.7, Q × f = 76,900 GHz and τf = + 3.3 ppm/°C were achieved. Zhang [12] added BaSnO3 to decline the sintering temperature of the ceramics system to 1450 °C, with excellent microwave performance of εr = 29.02, Q × f = 90,000 GHz and τf = 6.3 ppm/°C. Wang [13] reported that Ba1–xCax(Mg1/3Nb2/3)O3 (x = 0.005) ceramics could be sintered at 1500 °C, achieving good microwave performance (εr = 31.64, Q × f = 74,421 GHz and τf = 14.59 ppm/°C).
In this article, we tried to sinter BMN ceramics with B2O3 addition. By the addition of B2O3, we expected the ceramics system to lower the sintering temperature and improve the microwave dielectric performance. More significantly, the effect of B2O3 on phase development, microstructure and microwave dielectric performance of BMN ceramics were investigated in detail.
2 Experimental procedures
2.1 Specimen preparation
The samples were synthesized via the conventional solid-state reaction technique. For the synthesis of Ba(Mg1/3Nb2/3)O3 powders, BaCO3, MgO, Nb2O5 (purity ≥ 99%, ChengDu Kelong Chemical Co., Ltd, Chengdu, China) were mixed and ball-milled in a nylon jar with zirconia balls and deionized water for 16 h, and after drying, these powder were calcined at 1200 °C for 5 h. Then the calcined powders were re-milled for 18 h with x (x = 1–15) wt% B2O3 of ≥ 99% purity. Later, with 6wt% PVA as binder, the fine powders was pressed into cylindrical samples of 16 mm × 8 mm and then sintered at 900 °C for 5 h.
2.2 Characterization
The XRD analyses were carried out using a Philips X’Pert diffractometer within the 2θ range from 15 to 85° with CuKα radiation. The lattice parameters were calculated by the Rietveld refinement method using Jade. The apparent densities of sintered samples were measured by the Archimedes method. The relative density and porosity ratio were estimated as relative density (%) = D2/D1 × 100%, porosity (%) = (D1–D2)/D1 × 100%, respectively, where D1 and D2 are the X-ray theoretical density and the measured density, respectively. Microstructures of the samples were observed using scanning electron microscopy (SEM, FEI Inspect F) The cation ordering degree (S) of the specimens was obtained by the equation [14]: \(S = \sqrt {{{\left( {I_{100} {/}I_{102} } \right)_{{{\text{obs}}}} } \mathord{\left/ {\vphantom {{\left( {I_{100} {/}I_{102} } \right)_{{{\text{obs}}}} } {\left( {I_{100} {/}I_{102} } \right)_{{{\text{calc}}}} }}} \right. \kern-\nulldelimiterspace} {\left( {I_{100} {/}I_{102} } \right)_{{{\text{calc}}}} }}}\), in which (I100/I102)obs and (I100/I102)calc are the intensity ratio of the (100) superlattice reflection to that of (102) main reflection from the observed and the calculated values of complete ordering, respectively. The dielectric characteristics at microwave frequencies were measured by the Hakki-Coleman dielectric resonator method in the TE011 mode using a network analyzer (HP83752A) [15]. The sintered samples used for this study had the ratio: diameter/length = 1.8–2.2. The τf values were determined using the equation: τf = (ft2–ft1)/(ft1 × (t2–t1)), where ft1 and ft2 are the resonant frequencies at t1 = 25 °C and t2 = 85 °C, respectively.
3 Results and discussion
Generally speaking, the densification of the ceramics is mainly dependent on the sintering temperature. The relative densities of the samples are showed as a function of sintering temperature and B2O3 content in Fig. 1. The samples were sintered in the range from 850 to 950 °C for 5 h. For all samples, the highest relative density was always obtained at 900 °C and subsequently decreased with sintering temperature concentration. The increase of sinterability was due to the decrease of porosity and grain growth. At the same time, the reduction of sinterability was ascribed to the increase of porosity and the formation of secondary phase. Additionally, the relative density first increased to the maximum value and then dropped with increasing x. Similar results were observed in Ba(Mg1/3Nb2/3)O3 ceramics with BaWO4 addition [16], where the change of grain and porosity led to the variation of relative density. All in all, it was proved that the perfect sintering temperature of the samples remained to be 900 °C with the increase of x. Therefore, all B2O3-doped samples were sintered at 900 °C in this study.
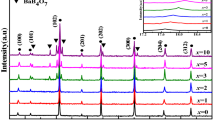
BMN ceramics with various B2O3 contents were sintered at 900 °C for 5 h, and Fig. 2 displays the XRD patterns of these samples. From the XRD patterns, we could find that the main phase of the samples was BMN phase with a complex perovskite crystal structure. For the samples with x ≤ 7, BMN peaks without secondary phases were observed, indicating the existence of a pure BMN phase. However, when x ≥ 10, peaks for the Ba2B2O5 phase appeared and the diffraction peak intensity of secondary phase was increased with B2O3 concentration. When increasing B2O3 content, no secondary phase expect Ba2B2O5 phase was found in the samples. When B2O3 was added to Ba(Mg1/3Nb2/3)O3 ceramic, B2O3 reacted with BaO, forming the Ba2B2O5 phase. A similar result was observed in the PbO–B2O3-added BaTiO3 and B2O3-added Ba5Nb4O15 ceramics [17, 18]. However, the BaB2O4 phase was not observed in B2O3-added BMN. As we know, the densification sintering temperature of BMN was very high (about 1550 °C), so it was difficult to sinter. However, BMN ceramics could be well sintered at 900 ◦C with B2O3 addition. Therefore, it was reasonably believed that the secondary phase containing BaO and B2O3 formed with B2O3 addition, which was beneficial to the improvement of sintering property of BMN ceramics.
The lattice parameters and unit cell volume of the BMN ceramics with x (x = 1–15) wt% B2O3 were calculated and the results are displayed in Fig. 3. With increasing the doping amount of B2O3 from 1 to 15 wt%, the lattice parameters and the unit cell volume were decreased. Meanwhile, the c/a ratio first increased to the peak value and then decreased. And the samples possessed the peak value in c/a ratio at x = 7. The most important indicator of the hexagonal distortion caused by the cation ordering was the c/a ratio in the BMN ceramics[16, 19]. According to previous work, the increase of c/a ratio was beneficial to improve the Q × f value of the ceramic system [20]. This study revealed that the perfect c/a ratio could be achieved for the samples with appropriate B2O3 addition.
Figure 4 displays the SEM photographs of the BMN ceramics with varying amounts of B2O3 sintered at 900 °C for 5 h. We can see that BMN ceramic with x < 7 sintered at 900 °C showed many pores and incompact microstructure, which could be explained that there were not enough liquid phases to fill the residual pores. When increasing B2O3 content, the microstructures of the samples were improved greatly, which was caused by the formation of liquid phases. Obviously, the abundant liquid phases arising from more B2O3 addition could accelerate the mass transportation in the sintering process, which contributed to the densification of the systems. When x = 7, the sample showed the best microstructure of all samples, suggesting that appropriate B2O3 addition in BMN ceramics could obtain a dense microstructure and excellent properties. Moreover, combining with Fig. 4 and Table 1, with the x value increasing from 1 to 7, the grain growth was faster and faster and the porosity declined gradually in the samples. Thus, it could be inferred that appropriate B2O3 addition in BMN ceramics could improve the densification of ceramics system. However, from the SEM images of the samples with x > 7, a considerable number of pores were obvious and the porosity increased sharply at the meantime. And the formation of pores could be caused by the grain growth with surplus B2O3 content addition.
For further determining the two crystalline phases in SEM images, both small and large grains in the samples (Fig. 4e–f) were measured by the energy dispersive X-ray spectrometer (EDS) testing. We once selected four points and the four testing results are shown in Table 2. Combining with EDS testing and the XRD analysis, the small grains (spot A and C) was the BMN phase. It was reported that, secondary phase [13] and excessive liquid phase [2] could restrain the grain growth. While the Mg elemental was relatively low as that of BMN, which was due to the volatilization of MgO during the high temperature sintering process [21]. The large grains (spot B and D) located at the liquid phase and the EDS testing indicated that the agglomeration of liquid phase was also Ba-Nb-based phase. As reported by our previous study [20], the small grains were wrapped by the secondary phase and the surplus liquid phase so as to form many large grains. Thus, it could be deduced that there was phase equilibrium among the ternary system B3+-liquid phase-Ba(Mg1/3Nb2/3)O3 [22]. The above results were good agreement with the XRD analysis, which revealed that surplus B2O3 addition could cause the appearance of the secondary phase Ba2B2O5.
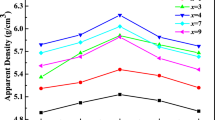
Figure 5 presents the apparent density and dielectric constant (εr) of BMN ceramics with x (x = 1–5) wt% B2O3 sintered at 900 °C for 5 h. Let’s first analyze the apparent density. When x ≤ 7, the apparent density of the samples was very low firstly and increased with increasing B2O3 content. As shown in Fig. 4a–d, the porous microstructure formed in the samples sintered at 900 °C changed to a dense structure with B2O3 concentration. Therefore, the increase of apparent density with B2O3 concentration was explained by the variation in the microstructures. When x ≥ 10, the apparent density began to decrease when large amounts of B2O3 were added. Associating with Fig. 4e and f, the dense microstructure was developed with moderate B2O3 addition, but the loose microstructures with a few residual pores formed when the B2O3 content increased. Therefore, the variation in apparent density with B2O3 was very closely related to the microstructure of the samples. The dielectric properties of all samples were tested at 1 MHz. Figure 5 illustrates the dielectric constant (εr) of the samples as a function of B2O3 addition. As we know, the dielectric constant were mainly related to the apparent density, phase composition and microstructure [23, 24]. As shown in Fig. 5, the dielectric constant (εr) increased gradually when x = 1–7, and then decreased when x > 7, showing the similar variation tendency as the apparent densities. Therefore, it could be inferred that the dielectric constant of the samples were closely related to the apparent density and located in the range of 29.3–31.6. Because of the dense microstructure, the sample with x = 7 sintered at 900 °C exhibited the highest dielectric constant (31.6). Meanwhile, the apparent density of the samples decreased with the surplus B2O3 addition, thus, the decrease of the dielectric constant could be attributed to the decrease in the density of the samples.
The correlations between the cation ordering degree (S) and the dielectric loss are shown in Fig. 6, which indicate that the cation ordering degree increase with B2O3 concentration, while the dielectric loss decrease, accordingly. That is, an inverse relationship existed between the cation ordering degree (S) and the dielectric loss from x = 1 to x = 15. According to the above analysis, the S value of the samples reached the maximum value at x = 7, and then decreased sharply with B2O3 concentration, which was the same with the changing tendency of c/a ratio. As reported by Tang [25], the dielectric loss was related to the cation ordering degree, phase composition and crystal defects. Especially, the cation ordering degree was very closely related to the dielectric loss of materials. Additional, many defects in the crystal lattice, such as lattice distortion, pores and lattice defect were also the key influences on the dielectric loss of ceramics. For the sample with x = 7, the dielectric loss decreased to 1.6 × 10−4, which was attributed to the reduction of pores and the increase of cation ordering degree. Thus, the dielectric loss decreased gradually at x ≤ 7 with B2O3 concentration. Moreover, combining with Fig. 2, the increase of dielectric loss was due to the formation of secondary phase Ba2B2O5.
The Q × f values and τf values of BMN ceramics with x (x = 1 - 15) wt% B2O3 sintered at 900 °C for 5 h are presented in Fig. 7. When increasing B2O3 content, the Q × f values first increased to the maximum value of 89,800 GHz with x = 7, and dropped thereafter. The Q × f values of the samples initially increased with B2O3 concentration, which was attributed to the increase in apparent density. When surplus B2O3 was added to the BMN ceramics, an inhomogeneous phase with secondary phase Ba2B2O5 was developed. Therefore, the decrease of Q × f values with increasing B2O3 content could be ascribed to the appearance of the secondary phase Ba2B2O5. Moreover, since the apparent density decreased with B2O3 concentration, the decline in Q × f values was also explained by the decrease of apparent density. As shown in Fig. 7, the variation of the temperature coefficient of resonant frequency (τf) was in accord with the proportion of B2O3. It was obvious that the τf values decreased first to 3.6 ppm/ °C (x = 7), and then increased to 15.8 ppm/ °C (x = 15) with the increase of B2O3 content. The τf values were determined by the additive content, secondary phase and the degree of densification [26, 27]. Associating Fig. 5 with Fig. 7, the τf value in Fig. 7 displayed the opposite changing tendency as that of apparent density in Fig. 5. Thus, it was reasonably believed that the increase of apparent density could be the key influence on the decrease in the τf value when x ≤ 7. However, the τf value began to increase to 15.8 ppm/°C with x further increased to 15. The variation of τf value showed a ring trend due to the decrease of density, which was ascribed to the surplus B2O3 addition.
As is known to all, BMN ceramics have attracted much attention due to their excellent dielectric properties. However, the main problem of the BMN sintering is that the densification sintering temperature is approximately 1550 °C, which is a great difficulty for the application of substrate materials. In this study, the sample with x = 7 could be well sintered at 900 °C for 5 h and showed good microwave dielectric properties: εr = 31.6, Q × f = 89,800 GHz, τf = 3.6 ppm/°C. Additionally, Table 3 presents the microwave dielectric properties of some typical Ba(Mg1/3Nb2/3)O3-based ceramics. As shown in Table 3, with the change of time, the evident progress has been made in improving microwave dielectric properties and sintering properties. The above results showed that B2O3 addition not only improved the microwave dielectric properties, but lowered the densification sintering temperature.
4 Conclusions
The BMN ceramics with x (x = 1–15) wt.% B2O3 were synthesized via the conventional solid-state ceramic route for studying the effect of B2O3 addition on the sintering behavior and microwave dielectric properties. The phase composition, microstructure and the microwave dielectric properties of BMN ceramics were studied as a function of B2O3 content. XRD test proved that the solid solution BMN was the main phase and Ba2B2O5 as the secondary phase. B2O3 worked as a sintering aid and improved the liquid phase sintering mechanism, which promoted noticeably the crystal growth and lowered the densification sintering temperature by approximately 650 °C. As increasing x from 1 to 7, the grain growth was promoted, moreover the τf values and dielectric losses dropped from 28.9 ppm/°C to 3.6 ppm/°C and from 9.2 × 10−4 to 1.6 × 10−4, respectively, and the Q × f values and cation ordering degree increased from 36,200 to 89,800 GHz and from 0.768 to 0.886, respectively. However, when the B2O3 addition was extremely excessive, with the increase of x up to x ≥ 10, the density, Q × f value and cation ordering degree decreased greatly, while the τf values and dielectric loss increased remarkably. At last, BMN ceramics with 7 wt% B2O3 sintered at 900 °C for 5 h displayed the excellent microwave dielectric properties of εr = 31.6, Q × f = 89,800 GHz, τf = 3.6 ppm/°C, which were expected to be used in microwave oscillator, mixer, detector and other devices.
Change history
12 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10854-021-06130-y
References
H. Shomoda, N. Ishitobi, K. Kawamura, M. Kobayashi, Jpn. J. Appl. Phys. 31, 3160 (1992)
E.Z. Li, S.X. Duan, S.M. Sun, H. Li, Y.A. Mi, X.H. Zhou, S.R. Zhang, J. Electron. Mater. 42, 3519 (2013)
H. Jantunen, A novel low temperature co-firing ceramic (LTCC) material for telecommunication devices, Ph.D. thesis, University of Oulu, Finland, 2001
B. Tang, Q.Y. Xiang, Z.X. Fang, X. Zhang, Z. Xiong, H. Li, C.L. Yuan, S.R. Zhang, Ceram. Int. 45, 11484 (2019)
S. Liu, B. Tang, M. Zhou, P. Zhao, Q.Y. Xiang, X. Zhang, Z.X. Fang, S.R. Zhang, Ceram. Int. 45, 8600 (2019)
S. Nomura, Ferroelectrics. 49, 61 (1983)
R. Freer, F. Azough, J. Eur. Ceram. Soc. 28, 1433 (2008)
N. Ichinose, T. Shimada, J. Eur. Ceram. Soc. 26, 1755 (2006)
Z.Q. Tian, H.X. Liu, H.T. Yu, S.X. Ouyang, Mater. Chem. Phys. 86, 228 (2004)
V. Singh, H.H. Kumar, D.K. Kharat, S. Hait, M.P. Kulkarni, Mater. Lett. 60, 2964 (2006)
Y. Kintaka, S. Kuretake, N. Tanaka, K. Kageyama, H. Takagi, J. Am. Ceram. Soc. 93, 1114 (2010)
J. Zhang, Y.Y. Zhou, Z.X. Yue, X.X. Zhang, L.T. Li, J. Am. Ceram. Soc. 98, 3942 (2015)
H. Wang, R.L. Fu, H. Liu, J. Fang, G.J. Li, J. Mater. Sci.: Mater. Electron. 30, 5726 (2019)
M.S. Fu, X.Q. Liu, X.M. Chen, Y.W. Zeng, J. Am. Ceram. Soc. 93, 787 (2010)
B.W. Hakki, P.D. Coleman, IEEE Trans. Microwave Theory Tech. 8, 402 (1960)
S. Peng, J.M. Xu, J. Mater. Sci.: Mater. Electron. 31, 22171 (2020)
Y. Kuromitsu, S.F. Wang, S. Yoshikawa, R.E. Newham, J. Am. Ceram. Soc. 77, 493 (1994)
D.W. Kim, J.R. Kim, S.H. Yoon, K.S. Hong, J. Am. Ceram. Soc. 85, 2759 (2002)
H. Li, P.C. Zhang, S.Q. Yu, H.Y. Yang, B. Tang, F.H. Li, S.R. Zhang, Ceram. Int. 45, 11639 (2019)
S. Peng, G.F, Luo, M.Q. Wu, S.Q, Yu, J.M. Xu, T.C. Huang, J.H. Zhou, J. Electron. Mater. 46, 2172 (2017)
K.P. Surendran, M.T. Sebastian, P. Mohanan, R.L. Moreira, A. Dias, Chem Mater 17, 142 (2005)
S.Q. Yu, B. Tang, X. Zhang, S.R. Zhang, X.H. Zhou, J. Am. Ceram. Soc. 95, 1939 (2012)
S.O. Yoon, S.H. Shim, K.S. Kim, J.G. Park, S. Kim, Ceram. Int. 35, 1271 (2009)
B. Li, Q.Y. Long, D.A. Duan, J. Mater. Sci.: Mater. Electron. 27, 2206 (2016)
B. Tang, Z.X. Fang, Y.X. Li, X. Zhang, S.R. Zhang, J. Mater. Sci.: Mater. Electron. 26, 6585 (2015)
S. Yu, B. Tang, S. Zhang, X. Zhou, J. Mater. Sci.: Mater. Electron. 24, 418 (2013)
C.H. Wang, X.P. Jing, L. Wang, J. Lu, J. Am. Ceram. Soc. 92, 1547 (2009)
J.H. Paik, S. Nahm, J.D. Bylin, M.H. Kim, H.J. Lee, J. Mater. Sci. Lett. 17, 1777 (1998)
Acknowledgements
This work was supported by the scientific Research Fund of Hunan Provincial Education Department (Grant No. 20A450), the project of the science and technology of Shaoyang City (Grant No. 2020GZ46), Science and technology innovation and entrepreneurship project of college students in Hunan Science and technology innovation talent plan (Grant No. 2020RC1011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, S., Yao, W., Zeng, W. et al. Effect of B2O3 on the microstructure and microwave dielectric properties of Ba(Mg1/3Nb2/3)O3 ceramics. J Mater Sci: Mater Electron 32, 11084–11091 (2021). https://doi.org/10.1007/s10854-021-05770-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05770-4