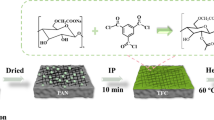
Abstract
In this paper, chitosan/nano-TiO2-modified liquid was coated on the surface of cellulose membrane by negative pressure suction filtration and a new method of preparing oil–water separation membrane was completed based on the pure nitrocellulose membrane. The performance parameters of cellulose composite membrane modified by chitosan and titanium dioxide with different concentration ratios were studied in terms of microstructure, element distribution and composition, wettability, using SEM, XPS, contact angle and other characterization methods. The optimal ratio of chitosan and titanium dioxide in the modified solution was selected to determine the optimal process parameters for the preparation of the new membrane. Because of the existence of nano-TiO2 particles in the new membrane, further study the effect of light on the membrane flux is required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Renewable biomass resources are widely distributed and abundant in nature. Cellulose, as one of the main components of biomass resources, is considered as one of the main substitutes of petroleum-based polymers [1, 2]. This substitute has a series of excellent characteristics such as biocompatibility, biodegradability and sustainable utilization. Cellulose is a kind of dehydrated glucose with direct chain connection. They have hierarchical structure, including aligned and misaligned chains, which make them superior to other materials in physical and chemical properties [3,4,5,6]. The molecular aggregation in cell structure increases their excellent material properties. Cellulose is widely used in experiments because of its large capacity, low cost, innocuity, easy modification and good interaction and reaction performance in chemical reactions. Cellulose is widely found in plants, algae and other biomass. Products containing cellulose are generally environmentally friendly and biocompatible.
Chitosan is the product of N-deacetylation of chitin, also known as deacetylation chitin. Chitosan is the product of natural polysaccharide chitin to remove part of acetyl group. It has many physiological functions such as biodegradation, biocompatibility and non-toxicity. It is widely used in many fields such as food additive, textile, medical fiber, medical dressing, drug development and other daily chemical industries. Chitin, chitosan and cellulose have similar chemical structures. Cellulose is hydroxyl at C2 position, chitin and chitosan are replaced by an acetyl amino group and an amino group at C2 position, respectively. Chitosan has a series of other unique properties, such as biodegradability, cell affinity and so on. Especially when chitosan contains free amino group, its various chemical reaction properties will become more active, and this kind of chitosan is the only basic polysaccharide in natural polysaccharide. The amino group in the molecular structure of chitosan is more active than the acetylamino group in chitin. Hence, chitosan has more excellent biological function and is easy to carry out chemical modification reaction. Therefore, chitosan is considered as a functional biomaterial with greater application potential.
In this study, chitosan/titanium dioxide-modified nitrocellulose composite membrane was prepared by vacuum filtration with different mass ratios of chitosan/titanium dioxide-modified solution. The material source of the membrane contains SiO2, chitosan and cellulose, and therefore it has the same chemical properties. Through the separation test of the emulsion, the following results can be obtained. When the membrane is completely blocked by the oil, the nano-titanium dioxide particles contained in the membrane will decompose the oil on the membrane surface into water or molecular groups by the way of photodegradation to clean the membrane surface and extend the service life of the separation membrane.
2 Methods and materials
2.1 Materials
Glacial acetic acid was used in the experiment, Kemat (Tianjin) Chemical Technology Co., Ltd.; deionized water, Tianjin Weike Biotechnology Co., Ltd.; chitosan, Tianjin xiensaopude Technology Co., Ltd.; glutaraldehyde, Beijing inokai Technology Co., Ltd.; pure nitrocellulose membrane, Tianjin Solomon Biotechnology Co., Ltd.; petroleum ether, Shanghai Anpu Experimental Technology Co., Ltd.; n-hexadecane, Tian He, a chemical laboratory of Tianjin University of science and technology.
2.2 Experimental equipment
Magnetic heating agitator, model 78-1 Changzhou sepu experimental instrument factory, electric agitator, model JJ-1A Changzhou Nuoji Instrument Co., Ltd., air drying oven, model DZF Shanghai Heng Scientific Instrument Co., Ltd., pure water machine, model GT-30 Changsha Keyi Instrument Co., Ltd., photochemical reaction device, model cel-lab500 Beijing Zhongjiao Jinyuan Technology Co., Ltd., scanning electron microscope, model JEOLJSM-6700, Japan JEOL company, X-ray photoelectron spectrometer, model thereto escalab 250xi, Thermo Fisher technology company of the United States, ultraviolet visible near infrared spectrophotometer, model UV-3600plus, Shimadzu company of Japan, contact angle meter, model OCA20, Germany DataPhysics company.
2.3 Experimental steps
2.3.1 Chitosan-modified nitrocellulose composite membrane
The first step is to use a pipette to measure 1 mL of glacial acetic acid solution and dissolve it in 99 mL of deionized water. A room temperature ultrasonic stirring device is used to stir for 5 min to make the solution mix evenly. Then the weight 20 mg of chitosan (CS) with deacetylation degree greater than 90% is measured and dissolved in the above-mentioned acetic acid solution, mixed with magnetic force for 30 min. Then the chitosan acetic acid solution is prepared. The second step is to use a pipette to measure 0.3 mL of 50% glutaraldehyde (GA), add into the chitosan solution, and mix with magnetic force for 10 h to obtain chitosan-modified solution. The third step is to measure 5 mL of the above chitosan-modified solution, add 5 mL of deionized water into it, mix evenly at room temperature, and then filter evenly to a pore diameter of 200 nm and a diameter of 40 by means of negative pressure suction filtration Mm pure nitrocellulose membrane (NC). Then, the obtained membrane was quickly put into a constant-temperature drying oven with a drying temperature of 35 °C. After drying in the oven for 5 h, it was evenly rinsed once with deionized water, and then dried for 2 h in a constant-temperature oven with the same temperature. Finally, the chitosan cellulose composite membrane was prepared and labeled as NCM. At the same time, the pure nitrocellulose membrane was labeled as PNC.
2.3.2 Chitosan/titanium dioxide-modified nitrocellulose composite membrane
In the preparation of chitosan/titanium dioxide-modified nitrocellulose composite membrane, the difference between the preparation procedure and NCM membrane lies in the third step as described in Sect. 2.3.1. In the third step, firstly, 5 mg, 20 mg and 35 mg titanium dioxide powder, respectively, were weighted and dissolved in 50 mL deionized water, dispersed with ultrasonic for 15 min to make them mix well. Secondly, a pipette is used to measure 5 mL of titanium dioxide solution of different concentrations, respectively, and put it into three clean beakers. Thirdly, 5 mL of chitosan-modified solution was added to the beakers so that the mixed solution in the beakers can be mixed evenly and then quickly pumped and filtered to the chitosan cellulose composite membrane. Fourthly, the obtained membrane was put into a constant-temperature drying oven quickly, and dried for 5 h at a drying temperature of 35 °C. After that, the film was evenly rinsed by deionized water once, and then dried in a drying oven at a constant temperature of 35 °C for 2 h. Finally, the chitosan/titanium dioxide-modified nitrocellulose composite films were labeled as NCTM (2:1), NCTM (2:4) and NCTM (2:7), respectively.
2.4 Emulsion preparation
Three different types of emulsified oils were prepared: sixteen alkane, petroleum ether and diesel emulsions, respectively. Three groups of control group were set up. One gram of three different oils were added to 1 L of deionized water respectively, and 0.1 g of twelve alkyl sulfonates (SDS) was added to the ultrasonic machine. The ultrasound was maintained for 1 h at room temperature, and then the solution was stirred for 3 h at 2800 rpm/min. The emulsion was obtained, which is emulsified oil needed for the experiment. The emulsified oil can be stable for several hours at room temperature.
2.4.1 Characterization method of emulsified oil separation experiment
The experiment of oil–water separation was carried out by solvent filter under negative pressure. The cellulose composite membrane was placed between the filter cup and the sand core, respectively. Before each oil–water separation experiment, deionized water was used to wet the cellulose composite membrane to ensure that the membrane was placed under water. The simulation of oily wastewater was realized by negative pressure filtration. In order to meet the experimental background of underwater super thin oil, all the cellulose composite membranes were dewetted and wetted by deionized water. They were placed between two glass devices and clamped with iron clamps. Then, the 50 mL O/W emulsion was poured into the filter cup, and the vacuum pump was opened to vacuum at 0.08 MPa. The oil–water separation test was carried out under this constant pressure. The effective area of the membrane is calculated as \(1.256 \times 10^{{{ - }3}} {\text{m}}^{2}\). After each oil–water separation experiment, the positive and negative sides of the membrane are washed twice with deionized water, and the next oil–water separation operation is carried out. Each type of oil–water separation experiment is repeated three times. The flux and oil removal rate of each experiment are measured and calculated, and the average value is calculated. The oil–water separation experiment can measure the filtration flux and oil removal rate, which can further verify the filtration performance of the membrane.
In the experiment, the filtration flux is expressed by Eq. (1).
where J is the filtration flux, unit: L/(m2 h); V is the solution volume, unit: L; A is the filtration area, unit: m2; and T is the filtration time, unit: h. After filtration, the content of oil in the filtrate was determined by ultraviolet spectrophotometry according to SL93.2–1994 and HJ970-2018 standards.
The calculation equation of oil removal rate in oil–water separation experiment is as follows:
In Eq. [2], \(\eta\) is the oil removal rate of the oily wastewater. C0 is the oil content of the original oily wastewater, unit: mg/L; C1 is the oil content of the oily wastewater after the oil–water separation experiment, unit: mg/L.
3 Results and discussion
3.1 SEM analysis of chitosan titanium dioxide composite film
The results show that the roughness of the surface structure and the hydrophilic groups or chemical bonds have a significant effect on the hydrophobic, anti-pollution and hydrophilic diffusion ability of the membrane materials [7,8,9]. Figure 1 is the SEM characterization of pure nitrocellulose membrane and cellulose-modified membrane under different conditions.
Through the analysis of Fig. 1a, it can be found that the pore diameter of pure nitrocellulose membrane is large, and the retention rate of small and medium-sized substances in oil-bearing wastewater is greatly reduced. Hence, this kind of emulsified oil cannot be effectively separated. According to the analysis of Fig. 1b, there is a layer of micro-nanomaterials on the surface. It can be concluded that these micro-nanomaterials are coated chitosan, which is a good hydrophilic material. Therefore, the wettability of the membrane is better than that of the pure nitrocellulose membrane. For the comparative analysis of Fig. 1c–e, it can be found that the surface roughness of the cellulose composite membrane is becoming larger and larger, and the membrane pore is gradually reduced. Hence, the inorganic nano-titanium dioxide particles can be coated on the pure nitric acid fiber membrane pore and the membrane surface. According to the theoretical analysis of Wenzel equation, the larger the surface roughness of the hydrophobic film material is, the larger the contact angle of the oil drop on its surface is. Therefore, the conversion of the surface of the cellulose composite film to the ultrahydrophobic film can well retain the oil in the water.
3.2 Analysis of surface wettability of chitosan titanium dioxide film
The effect of membrane on oil–water separation mainly depends on its wettability. Especially the hydrophilicity of membrane surface and the super hydrophobicity under water are very important for oil–water separation. The water contact angle (WCA) and the oil contact angle (OCA) are usually used to evaluate the membrane wettability. In this paper, the wettability of pure nitrocellulose and various kinds of modified cellulose composite membranes WCA and OCA were measured to analyze their wettability, and then the composite membrane with the best wettability could be selected for the simulated oil–water separation experiment.
3.2.1 Analysis of contact angle characterization results
As shown in Fig. 2a, compared with that of pure nitrocellulose membrane, the contact angle (WCA) of water in the air of pure nitrocellulose membrane is 97.7 ± 5°, and the WCA of chitosan-modified nitrocellulose membrane is 61.2 ± 4°. At the same time, the WCA of NCTM (2:1) membrane is 32.6 ± 3.5°, and the WCA of NCTM (2:4) membrane and NCTM (2:7) membrane is 0° with TiO2 in the modified solution. The increasing mass and the amount of TiO2 loaded to the cellulose membrane form more rough structures on the surface of the membrane. Nano-TiO2 had excellent hydrophilic inorganic materials, which greatly reduced the WCA value of the chitosan/TiO2 cellulose composite membrane, and made the performance of the super water affinity.
As shown in Fig. 2b, it can be seen that the OCA of pure nitrocellulose membrane is 86.3 ± 2.6° and that of chitosan-modified cellulose composite membrane is 127.6 ± 3.1°. This is formed by the formation of rough micro-nanostructure on the membrane surface by the active amino and hydroxyl groups in chitosan and its chitosan-modified solution. The oil repellency of the membrane, however, is weak under water, which is not suitable for oil–water separation experiment. The OCA of NCTM (2:1), NCTM (2:4) and NCTM (2:7) films is 148.9 ± 3°, 153 ± 2.5° and 156.2 ± 3.3°, respectively. The hydrophilicity of the film surface and the micro-nanostructure of the film surface are improved with the application of nano-TiO2 to the cellulose film. The results show that the composite membrane shows super hydrophobicity under water when the mass ratio of chitosan to titanium dioxide is 2:4 and the mass ratio of chitosan to titanium dioxide is 2:7. Based on the comprehensive analysis of WCA and OCA of different cellulose composite membranes, it can be found that the cellulose composite membranes with the mass ratio of chitosan to titanium dioxide of 2:4 and 2:7 have good super hydrophilic and super hydrophobic properties, which are suitable for oil–water separation experiments.
It can be concluded from the data that the characteristics of super hydrophilic and super hydrophobic oil on the membrane surface are determined by the rough hierarchical microstructure and the chemical composition of the membrane surface [10, 11]. In this study, the WCA and OCA values of pure nitrocellulose membrane and chitosan-modified cellulose composite membrane were compared. It was found that the amino and hydroxy hydrophilic groups in chitosan improved the wettability of the membrane surface, and the rough microstructure of chitosan-modified cellulose composite membrane improved significantly, which further improved the wettability of the membrane.
3.3 X-ray photoelectron spectroscopy analysis of CS/TiO2 composite films
The X-ray photoelectron spectroscopy was used to determine the energy spectrum of the related series of cellulose composite films, and the changes of chemical composition and structure on the surface of the membrane were analyzed.
The XPS spectra of different cellulose composite films are shown in Fig. 3. As shown in Fig. 3a, the XPS spectra of different cellulose composite films show that O, Ti, N and C have corresponding absorption peaks. On the surface of pure nitrocellulose nitrate membrane and cellulose composite membrane, C (283.98 eV), o-element (532.9 eV) and N element (407.3 eV) can be detected on the surface of pure nitrocellulose nitrate membrane and cellulose composite membrane with different modifications. The N element is found on pure nitrocellulose and chitosan. The N-element content in chitosan is smaller than that of N in pure cellulose nitrate film. It can be seen from the figure that the relative content of N is lower than that of pure cellulose nitrate membrane, indicating that chitosan is applied to the surface of cellulose membrane. In addition, it can be seen that Ti2P has a strong absorption peak, and Ti2P increases with the increase of TiO2 content in the modified mixture. The stronger the absorption peak, the more successful the surface of cellulose film was loaded onto the nano-TiO2 particles. As shown in Fig. 3b, there are three apparent absorption peaks at 287.09ev, 286.1 eV and 283.9 eV, respectively, corresponding to the -CHx (C–C bond and C–H bond), C=N bond and C–O bond. The C=N bond in the spectrum shows that the cross-linking of chitosan with glutaraldehyde makes the amino group and aldehyde group cross-linking reaction. As shown in Fig. 3c, there are two distinct characteristic peaks at 463.6ev and 457.4 eV of different cellulose films, which indicates that the binding energy of Ti2p1/2 and Ti2p3/2 is 463.6ev and 457.4ev, respectively. As shown in Fig. 3d, the high-resolution N1s spectrum of different cellulose films corresponds to the characteristic peak of N1s at 457.4 eV. With the coating of titanium dioxide and chitosan on pure cellulose membrane, the content of N decreases gradually, and the characteristic peak of n1s decreases obviously.
3.4 Stability analysis of chitosan titanium dioxide composite membrane
In fact, the composition of oil-bearing sewage is complex and difficult to separate, and a large amount of oil-bearing sewage is in a strong acid–base and high salinity environment. If the membrane separation technology is used to treat this type of oil-bearing sewage, there will be higher requirements for the acid and alkali resistance and high salinity resistance of membrane materials.
It is known from the above discussion that the prepared NCTM (2:4) membrane is the best formula proportion of oil–water emulsion separation. Therefore, in order to explore the stability of NCTM (2:4) membrane prepared under strong acid base and high salinity environment, the PH 1, PH 13 and mass fraction of 5% NaCl diesel oil emulsion were, respectively, prepared by hydrochloric acid, NaOH and NaCl. Under the conditions of pH 1, pH 13 and mass fraction of 5% NaCl, the filtration flux of NCTM (2:4) membrane is 3507.9 L/(m2 h), 4218.3 L/(m2 h), 4065.7 L/(m2 h) and the oil removal rates are 98.4%, 98.7% and 98.2%, respectively, by ultraviolet spectrophotometry. The specific situation is shown in Fig. 4.
In order to better study on the filtration stability and pollution resistance of modified cellulose composite membrane, high-throughput, high oil removal rate and excellent infiltrative NCTM (2:4) membrane were used to verify and elaborate. In the test of diesel oil emulsion separation cycle using NCTM (2:4) membrane, the filtration flux of each filtration test can be shown in Fig. 5. In addition, the oil removal rate of NCTM (2:4) membrane in the tenth oil–water separation test was still high, reaching 98.5%.
In order to directly test the separation ability of NCTM (2:4) membrane, NCTM (2:4) membrane was immersed in 50 mL deionized water and put into a negative pressure filtration device. An appropriate amount of oil red solution was added to the O/W emulsion of diesel oil and oil red diesel emulsion was added to the filter cup of the negative pressure filter. After opening the vacuum pump switch, the water in the oil quickly penetrated into the cone bottle. The oil red and diesel oil droplets were trapped on the membrane surface. The visual oil–water separation test process is shown in Fig. 6a–c to compare with the emulsion before separation. The filtrate is transparent under the optical microscope, without any contaminants, and there is no oil droplet.
Intuitive oil–water separation process (where a is that the membrane is wetted by water before oil–water separation; b is that 50 mL of oil red solution is added to the device; c is the state diagram after oil red solution filtration); d is an optical microscope comparison of diesel O/W emulsion and filtered filtrate
3.5 Analysis of photocatalytic properties of composite membrane
3.5.1 Overview of titanium dioxide photocatalysis
Photocatalysis technology is gradually rising in the 1970s. Photocatalyst can convert light energy into chemical energy under the condition of light and accelerate the redox reaction between materials, which is a new purification technology. Nanoparticle-assisted photocatalysis technology is used to remove organic pollutants from industrial and domestic sewage. There are various "sensitizers" that can accelerate the process. This process is a combination of heterogeneous catalysis and solar energy technology [12, 13]. Photocatalytic processes decompose alcohols, carboxylic acids, amines, herbicides and aldehydes into carbon dioxide, water and simple inorganic acids [14]. Titanium dioxide is a common photocatalyst. The wide bandgap energy of titanium dioxide can stimulate electrons to produce hydroxyl radicals in ultraviolet energy, which is the key to photodegradation of pollutants.
In this paper, titanium dioxide nanoparticles are coated on the surface of pure nitrocellulose membrane by coating method. This technology has the advantages of simple operation and high working efficiency. Compared with other methods (physical method, chemical method and physical–chemical combination method) [15, 16], it is faster, more economical and controllable. In this paper, through the configuration of different concentration ratios of modified solution, the purpose is to achieve the cleaning of the membrane surface and prolong the service life of the membrane.
3.5.2 Analysis of oil pollution on cellulose membrane by photocatalysis
In order to explore the photocatalytic effect of nano-TiO2 particles loaded in NCTM (2:4) membrane on oil pollution, seven cellulose composite membranes with membrane flux less than 400 L/(m2 h) after 12 times of oil–water separation operation were prepared. The NCTM (2:4) membrane is vertically placed in a beaker filled with a proper amount of deionized water. The surface of the contaminated membrane is to face the UV light source and then the beaker is placed in the photocatalytic reaction device produced by Beijing Zhongjiao Jinyuan Co. Ltd. The light source of the device is placed in the middle of the box body and irradiated by the UV light source of the mercury lamp. For the surface of NCTM (2:4) membrane polluted by oil pollution, due to a lot of heat produced by mercury lamp, the light source switch is turned on and the cold water valve is timely turn on to ensure the normal operation of the cooling circulation system. Each NCTM (2:4) membrane polluted by oil pollution is place one by one in the ultraviolet light for 3 h, 6 h, 9 h, 12 h, 15 h, 18 h and 21 h. The process of photocatalytic reaction device and its photocatalytic composite membrane are shown in Fig. 7.
After the light is over, the separation test of diesel O/W emulsion is carried out by using negative pressure filtration device. The flux and separation rates of different light time films are calculated as shown in Fig. 8. The results show that the photocatalytic effect of titanium dioxide has a certain effect on the degradation of oil contamination on the surface of the polluted membrane. Before 15 h of illumination, the filtration flux of the corresponding membrane increases with an increase of illumination time, which indicates the oil contamination on the surface of the blocked membrane is degraded into H2O and CO2 by TiO2. For the intermediate organic matter of oil pollution with smaller particles, the accumulation of oil pollution in the internal pores of the membrane is reduced, and water molecules are easier to penetrate the membrane surface. As a result, the filtration flux is improved obviously. The oil removal rate of the membrane, however, is slightly reduced due to TiO2. The photocatalytic degradation process is not thorough enough, and some large particles of oil are transformed into small particles of oil organics, which has a negative impact on the content of filtrate oil. The results show that TiO2 on the membrane surface and inside the membrane pore can photocatalyst diesel oil to accumulate oil, and reasonable utilization of TiO2 can prolong the life of the membrane, which can be used in practical engineering.
4 Conclusions
In this experiment, chitosan-modified solution and chitosan/titanium dioxide-modified solution with different mass ratios were coated on the surface of hydrophobic pure cellulose nitrate membrane by negative pressure suction filtration, which turned it into super hydrophilic in the air and super hydrophobic in the water. The special wettability of pure cellulose nitrate membrane and CS: TiO2 Composite membrane with different mass ratios was studied. SEM, contact angle and other characterization methods were used to systematically study the properties of different cellulose composite membranes. The microstructure, elemental distribution and composition of the cellulose composite membranes were compared. The optimum mass ratio of 2:4 for O/W diesel oil emulsion filtration and oil removal rate was through different cellulose composite membranes.
In this paper, chitosan-modified solution and chitosan/TiO2-modified solution with different mass ratios were prepared, respectively. Different types of modified solution were evenly filtered onto the surface of hydrophobic pure nitrocellulose membrane by negative pressure filtration method, and then the corresponding cellulose composite membrane was prepared. According to the indexes of wettability, filtration flux, oil removal rate and cycle times, the cellulose composite membranes with the best comprehensive performance were selected from different series of ratios. NCTM (2:4) membrane, pure nitrocellulose, chitosan and titanium dioxide have the advantages of low cost and environmental protection. In addition, the membrane filtration of oil–water mixture has the advantages of simple operation and no secondary pollution caused by dosing. The cellulose composite membrane prepared has great potential in engineering application. However, further research is needed to put it into practical production smoothly. The following research can be carried out from the following aspects:
-
(1)
Further expand the research gradient, optimize the preparation conditions, and prepare a cellulose composite film with low cost, environmental protection, high oil removal rate, corrosion resistance and stability.
-
(2)
In order to further study the specific effect of the ratio of various substances in the modified solution on the membrane thickness, we can establish relevant models to analyze and discuss. The specific effect of the membrane thickness on the filtration flux of this type of membrane can be further studied.
References
J.L. Wertz, J.P. Mercier, O. Bedue, Cellulose Science and Technology (CRC Press, Boca Raton, 2010).
H. Zhu, W. Luo, P.N. Ciesielski et al., Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 116(16), 9305–9374 (2016)
D. Trache, M.H. Hussin, M.K.M. Haafiz et al., Recent progress in cellulose nanocrystals: sources and production. Nanoscale 9(5), 1763–1786 (2017)
T. Rosenau, A. Potthast, H. Sixta et al., The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 26(9), 1763–1837 (2001)
Y. Habibi, L.A. Lucia, O.J. Rojas, Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 110(6), 3479–3500 (2010)
Y. Li, M. Lin, J.W. Davenport, Ab initio studies of cellulose I: crystal structure, intermolecular forces, and interactions with water. J. Phys. Chem. 115(23), 11533–11539 (2011)
J. Li, L. Yan, W. Hu et al., Facile fabrication of underwater superoleophobic TiO2 coated mesh for highly efficient oil/water separation. Colloids Surf. A 489, 441–446 (2016)
Q. Liu, A.A. Patel, L. Liu, Superhydrophilic and underwater superoleophobic Poly(sulfobetaine methacrylate)-grafted glass fiber filters for oil-water separation. ACS Appl. Mater. Interfaces. 6(12), 8996–9003 (2014)
Y. Xiang, F. Liu, L. Xue, Under seawater superoleophobic PVDF membrane inspired by polydopamine for efficient oil/seawater separation[J]. J. Membr. Sci. 476, 321–329 (2015)
X. Zhao, Y. Su, Y. Liu et al., Free-standing graphene oxide-palygorskite nanohybrid membrane for oil/water separation. Acs Appl. Mater. Interfaces 8(12), 8247–8256 (2016)
S. Huang, R.H.A. Ras, X. Tian, Antifouling membranes for oily wastewater treatment: interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 36, 90 (2018)
Y.K. Abdel-Monem, Efficient nanophotocatalyt of hydrothermally synthesized anatase TiO2 nanoparticles from its analogue metal coordinated precursor. J. Mater. Sci.: Mater. Electron. 27(6), 5723–5728 (2016)
Y.K. Abdel-Monem, S.M. Emam, H.M.Y. Okda, Solid state thermal decomposition synthesis of CuO nanoparticles from coordinated pyrazolopyridine as novel precursors. J. Mater. Sci.: Mater. Electron. 28, 2923 (2017)
M. Madkour, Y.K. Abdel-Monem, F. Al-Sagheer, Controlled synthesis of NiO and Co3O4 nanoparticles from different coordinated precursors: impact of precursor’s geometry on the nanoparticles characteristics. Ind. Eng. Chem. Res. 55, 12733 (2016)
A. Bumajdad, M. Madkour, Y. Abdel-Moneam et al., Nanostructured mesoporous Au/TiO 2 for photocatalytic degradation of a textile dye: the effect of size similarity of the deposited Au with that of TiO2 pores. J. Mater. Sci. 49(4), 1743–1754 (2014)
M. El-Kemary, Y. Abdel-Moneam, M. Madkour et al., Enhanced photocatalytic degradation of Safranin-O by heterogeneous nanoparticles for environmental applications. J. Lumin. 131(4), 570–576 (2011)
Funding
This study was funded by Innovative Research Group Project of the National Natural Science Foundation of China (Grant No. 20024).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Chen, H., Wang, Q. et al. Further modification of oil–water separation membrane based on chitosan and titanium dioxide. J Mater Sci: Mater Electron 32, 4823–4832 (2021). https://doi.org/10.1007/s10854-020-05221-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-05221-6