Abstract
Low sintering temperature and good temperature stability are the crucial parameters for the actual application of the dielectric capacitors. In this work, lead-free relaxor ferroelectric ceramics with chemical formula (1 − x)(Ba0.4Sr0.6)TiO3-xBi(Zn2/3Nb1/3)O3 [(1 − x)BST-xBZN, (x = 0.00 to 0.225)] were developed through a solid-state synthesis route. The microstructures, dielectric performance, and temperature stability were studied in detail. The results show that 0.775BST-0.225BZN bulk ceramic with capacitance-temperature dependence satisfied with X8R specification can be obtained at a sintering temperature of 1140 °C. In addition, the energy storage performance of 0.775BST-0.225BZN bulk ceramic exhibits good temperature stability in a wide range of temperatures from 25 to 150 °C. High dispersion results in capacitance-temperature stability and energy storage stability. More importantly, the 0.775BST-0.225BZN ceramic also displays good charge–discharge performance dependence on temperatures (variations of the current density and power density are less than 3% over 25–150 °C). These results demonstrate that 0.775BST-0.225BZN lead-free ceramic is a potential material for dielectric capacitors that can be operated in a wide range of temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, pulsed power capacitors have been widely developed and applied in the energy storage industry. With the increasing demands of energy storage applications, the requirement for pulsed power capacitors with excellent dielectric properties has become an urgent task. Compared with chemical energy storage devices and superconducting magnetic energy storage systems, dielectric capacitors play a crucial role in pulsed power equipment on account of the excellent dielectric properties. Chemical energy devices, such as batteries, possess high energy density but low power density, while the dielectric capacitors exhibit the opposite features. The electrochemical capacitors have medium energy density and power density. Moreover, dielectric capacitors are more suitable for high-voltage, low-cost, and large-scale applications. While the electrochemical capacitors can only suffer from the low operating voltage (< 3 V), large leakage current, and high cost [1,2,3,4,5] Moreover, they could be applied in a wide range of temperatures due to their good stabilization of the capacitance-temperature [4].
Generally, lead-based ceramics present excellent dielectric properties [6, 7], such as large polarization and high breakdown strength (BDS), but their possible toxicity limits their applications in the future. Thus, the lead-free ceramics gradually attracted the attention of researchers owing to the environmental friendliness, high BDS, and a relatively low dielectric loss. Among them, perovskite BaxSr1−xTiO3 (BST) comes from SrTiO3 (ST) and BaTiO3 (BT) which shows excellent properties, for example, low hysteresis loss and a relatively high energy storage density. To satisfy the growing demands of the dielectric capacitors, especially the ceramic capacitors, studies have aimed at improving the performance of the energy storage [6, 8,9,10,11,12,13,14,15]. For instance, Ba0.4Sr0.6TiO3 ceramics were reported in many publications showing excellent dielectric properties, such as good dielectric stability and moderate permittivity, for the application of energy storage equipment [1, 12, 15,16,17,18,19]. The (Sr0.4Ba0.6)0.925Bi0.05TiO3 ceramics have been reported with high dielectric constant and high tunability that were sintered for 4 h at 1260 °C [1]. Furthermore, Diao et al. have reported that Ba0.4Sr0.6TiO3-0.45wt% SiO2 ceramics sintered for 2 h at 1330 °C showed excellent properties of the energy storage density (Wst) and the release efficiency (η) at 134 kV/cm [19]. Additionally, it was reported that the (Ba0.4Sr0.6)TiO3 ceramics with grain size on the order of 0.5 μm possess a BDS of 243 kV/cm, much higher than that of ceramics with the grain size of 5.6 μm, which is on the order 114 kV/cm [13]. W.B. Li et al. have reported that 0.9BaTiO3-0.1Bi(Mg2/3Nb1/3)O3 ceramics modified by 0.3wt% MnCO3 and the 0.85BaTiO3-0.15Bi(Zn2/3Nb1/3)O3 ceramic exhibited good energy storage properties, respectively [20, 21]. Other approaches were also applied to improve the dielectric performances, such as thermal annealing [17], spark plasma sintering [10], and grain size controlling [15].
The previous reports indicated that excellent energy storage density and high BDS of BST-based ceramics have been achieved. However, BST-based ceramics with excellent properties of Wst and η still require the support of high sintering temperatures. High sintering temperature means large energy consumption. Thus, Reducing the sintering temperature of ceramics not only can save energy, but also lower the cost. Besides, the temperature stability cannot be ignored due to the harsh working environment of the dielectric capacitor. However, few works are focused on the temperature stability and lowering the sintering temperature for BST-based ceramics.
BT-Bi(Me)O3, BST-Bi(Me)O3 (Me = (Zn1/2Ti1/2)3+, (Mg1/2Ti1/2)3+, etc.) ceramics have been reported widely due to Bi3+ can improve the dielectric performances, and Me ions are adjustable [14, 21, 22]. In this work, Bi(Zn2/3Nb1/3)O3 has been selected to improve the characteristics of the Ba0.4Sr0.6TiO3 ceramics. On the one hand, the introduction of Bi3+ not only can effectively reduce the sintering temperature but also improve the temperature stability of the BST ceramics. On the other hand, an appropriate amount of incorporation with ZnO and Nb2O5 can reduce the sintering temperature and adjust the microstructure of the BST ceramics.
In this work, lead-free (1 − x)BST-xBZN, x = 0.00 to 0.225, bulk ceramics were synthesized via the solid-state route. Moreover, the microstructures, dielectric performances, and temperature stability of the prepared ceramics were discussed systematically. The capacitance-temperature dependence satisfied with X8R specification (from − 55 to 150 °C, the capacitance varies within ± 15%, which is defined by the Electronics Industry Alliance.) and sintering temperature of 1140 °C was obtained in 0.775BST-0.225BZN lead-free ceramic.
2 Experimental procedure
A series of lead-free (1 − x)BST-xBZN bulk ceramic samples were synthesized through the traditional solid-state synthesis route. High-purity powers(> 99.9%) were used as the raw materials including BaCO3, SrCO3, TiO2, Bi2O3, ZnO, and Nb2O5. According to the stoichiometric formula of (1 − x)(Ba0.4Sr0.6)TiO3-xBi(Zn2/3Nb1/3)O3 [(1 − x)BST-xBZN, (x = 0.00 to 0.225)], the powders were mixed and milled for 6 h in a polyurethane tank with zirconia balls and ultrapure water as the solvent. After the slurry drying, the obtained dried powders were calcined for 3 h at 1000 °C. Whereafter, with the same conditions as described above, the powders were ball-milled again for 24 h. Subsequently, a 5wt% solution of PVA was mixed with the obtained powders, which was subsequently pressed into cylinders under the pressure of 250 MPa. Finally, the pellets were put into a closed alumina crucible with powders having the same composition as the samples to diminish the evaporation of volatile elements, and sintered for 2 h at 1140–1250 °C after burning off the PVA adhesive.
Structures were researched via the X-ray diffraction (DMAX, Netherlands). The local structure distortions were recorded by Raman scattering spectrometer (Renishaw, London, UK) at 100 cm−1 to 1000 cm−1. Using SEM (Carl Zeiss NTS GmbH, Germany) to observe the ceramics for their surface morphologies. The temperature dependence of dielectric properties was recorded by an impedance analyzer (WAYNE KERR 6500B, UK) equipped with a temperature control system (VDMS2000, China). The P-E loops of all ceramics, which can be recorded by a ferroelectric test system (TF analyzer 2000, USA). The discharge current waveforms were obtained by an RLC discharge circuit test system with an oscilloscope (Tongguo (TG) technology, Pulsed Charge–discharge System, CFD-003).
3 Results and discussion
3.1 Phases and microstructure
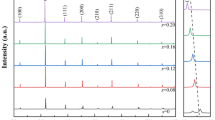
Figure 1 displays the XRD patterns for the prepared ceramics. Figure 1a exhibits that the main diffraction peaks are assigned to the perovskite pseudocubic phase, and the second phase (JCPDS#28-1243, SrNb2O6) can be observed when the content of BZN level up to 0.15. A solid solution was obtained as the content at x < 0.15, and then a new phase SrNb2O6 appears while x ≥ 0.15. Chen et al. revealed that the A-site ions were occupied by Bi3+, on account of the similar ion radius between Bi3+ and Ba2+, and then a finite solid solution in (Sr0.4Ba0.6)1–1.5xBixTiO3 can be formed [1]. Obviously, the appearance of the new phase SrNb2O6 is attributed to exceeding of the solid solubility limit.
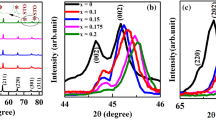
Figure 1b shows that the (200) diffraction peaks display a gradually shifting to the low angle as the BZN content increases, which manifests the large interplanar spacing. The coordination numbers of the cations (Ba2+, Sr2+, Bi3+) and the ions (Ti4+, Zn2+, Nb5+) are 12 and 6, respectively. Most likely, the Zn2+ and Nb5+ ions are probably entering into the B-sites to substitute for Ti4+ ions (0.0605 nm). In addition, Ba2+ ions (0.161 nm) and Sr2+(0.112 nm) of 12-fold coordination at A-sites are substituted by Bi3+ ions (0.144 nm). Obviously, the substitution can occur only between those ions with a similar radius.The effective radius of (Zn2/3Nb1/3)3+ is counted as r = (2/3)r(Zn2+) + (1/3)r(Nb5+) = 0.0707 nm (the ionic radius of Zn2+, Nb5+, and Ti4+ is 0.074, 0.064, 0.0605 nm, respectively) [2, 3]. To further study the crystal structure transformation caused by (Zn2/3Nb1/3)3+ substitution, Raman spectra at 100–1000 cm−1 were carried out.
Figure 2 plots the Raman spectra measured at the range from 100 to 1000 cm−1. The main peaks of Raman spectra are located at above 600 cm−1, 400–600 cm−1, 200–400 cm−1, and below 200 cm−1. For perovskite structure, the peaks under 200 cm−1 are induced by the vibrations of the ions at A-sites (Ba2+, Sr2+, Bi3+) [12, 23]. It is found the peak at ~ 148 cm−1 that can be vested in the vibration of Sr–O and Ba–O bonds decreases in intensity with the increases of x value [24]. The peak at about 190 cm−1, which means that some Sr2+ have been substituted by Bi3+ and Ba2+ [25]. For Bi3+-doped perovskite ceramics, the disorder of the A-sites could be increasing, and then the relaxor behavior appears [25,26,27]. According to the above discussion, it can be believed that the peaks of ~ 190 cm−1 reveal a part of the relaxor source for the inequitable substitution at the A-site. The vibration of B-O is related to the A1 (TO2) mode at ~ 270 cm−1. The A1 (TO3) mode at ~ 516 cm−1 within the scope of 400–600 cm−1 is caused by the vibrations of the TiO6 octahedron [24]. As the content of x increases, it is found that the peaks at ~ 516 cm−1 progressively become widened. This phenomenon is raised by the short-range distorted TiO6 octahedron induced by the occupation of Zn2+ and Nb5+ ions for Ti4+. Moreover, the peaks at 780 cm−1 are on account of the vibration of A1 (LO3) and E (LO) overlapped modes [7]. The result of the Raman spectra is well consistent with the above analysis of XRD.
Figure 3 shows the surface morphologies and the energy-dispersive X-ray spectroscopy (EDAX) for the samples. It can be seen that a few pores were observed on the surface of pristine BST ceramic in Fig. 3a. As shown in Fig. 3b, a dense microstructure and the second phase can be observed in the 0.775BST-0.225BZN ceramic. It can be seen that the grain size of (1 − x)BST-xBZN ceramics increases with increasing the value of x. The obvious change in the microstructure might be related to the fact that A-site and B-site elements such as Bi3+ and Zn2+ in (1 − x)BST-xBZN ceramics volatilize inevitably due to their low melting points [7], leading to the presence of oxygen vacancies, which is beneficial to mass transportation during sintering [22, 28]. From Fig. 3c and d, the EDAX analyses of the samples show that the 0.775BST-0.225BZN ceramic exhibits the cooccurrence of the BST phase with the second SrNb2O6 phase, which accord with the consequence of the XRD patterns.
From the above analyses, it can be concluded that the introduction of BZN modified the microstructure. The electrical property may be affected by the value of BZN. The change of the microstructure will further influence the dielectric performance of the (1 − x)BST-xBZN samples. Next, the dielectric performances were studied in detail.
3.2 Dielectric performances analysis
The dielectric performance dependence on temperature (− 140 °C to 150 °C) for all samples was recorded at the test ranges of 1 kHz–1 MHz, and the results are presented in Fig. 4. It can be observed that the permittivity has a deeply decreased with the BZN content increased, which can be attributed to that the BZN possesses a lower permittivity than that of the pristine Ba0.4Sr0.6TiO3 ceramics [22]. Obviously, with the increase of BZN content, Tm moves to a low direction at first and then shifts toward high temperatures. The dielectric characterization of all composition revealed a gradual change from normal ferroelectric behavior to high diffusive relaxor-like characteristics, which may be explained by the weak orbital hybridization of Mg–O destroyed the long-range Coulomb potential in the crystal. Furthermore, as reported, the Curie temperature of SrNb2O6 is around 300 °C, which may contribute to the increase of the Tm of the (1 − x)BST-xBZN samples [29]. The dielectric property of all ceramics means a gradual high diffusive relaxor-like behavior as the content of BZN increases. This phenomenon may be explained via the disorder of A-sites are substituted by Bi3+ and Ba2+ [30]. On the other hand, the relaxor behavior can also be explained via the long-range dipole action that was destroyed by the Ti4+, Zn2+, Nb5+ with different charges at B-site [31]. It should be noted that the dielectric constant and dielectric loss exhibit strong frequency dispersion behavior and the dielectric constant plateau of the ceramics. Therefore, it is rationalized that (1 − x)BST-xBZN ceramics are a typical relaxor ferroelectrics [32]. As the increase of BZN content, the curve becomes more and more gentle in the tested temperature ranges. This phenomenon indicates the optimization of temperature stability. Next, for all of the samples, the capacitance-temperature variations and the relaxation characteristics are further investigated.
The graphs of capacitance variation with temperature are given in Fig. 5a. The samples show good temperature stability with the increasing content of BZN. When the BZN content is 0.225, the capacitance-temperature variation satisfies the X8R specification (from − 55 to 150 °C, the capacitance varies within ± 15%, which is defined by the Electronics Industry Alliance.). The generation of the SrNb2O6 (Tm ~ 300 °C) can influence the optimization of dielectric temperature characteristics, which flats the Tm peaks of the samples.
A Curie–Weiss formula [7, 24, 27] was introduced to further study the diffuse character of the (1 − x)BST-xBZN ceramics, as follows:
C represents the Curie constant. Where \({\varepsilon }_{\gamma }^{\prime}\) represents the permittivity at the temperature of T and \({\varepsilon }_{m}^{\prime}\) is the peak value of permittivity, respectively. Tm represents the corresponding temperature at the maximum value of the permittivity [24, 33]. The γ represents the diffusion index. γ = 1 and γ = 2 represents a typical ferroelectric and a complete disorder, respectively. When 1 < γ < 2, the typical relaxation behavior accompanied by the dispersion phase transition is found. The value of γ is obtained by fitting the test data at 1 kHz through Eq. (1). It can be observed that the value of γ for all ceramics increased from 1.22 to 1.56, 1.59, 1.68, 1.70, 1.76 with the BZN content increased from 0.00 to 0.225. This phenomenon indicates the typical transition to relaxor ferroelectric from ferroelectric, which is beneficial to improving the energy storage efficiency and reducing the remanent polarization of the ceramics.
The P-E hysteresis loops of the specimens were recorded under an electric field of 170 kV/cm at a tested frequency of 10 Hz as shown in Fig. 6a. The value of the remanent polarization (Pr), energy storage density (Wst), maximum polarization (Pmax), releasable efficiency (η) is listed in Table 1. As shown in Fig. 6a, the Wst of the 0.95BST-0.05BZN ceramic is 0.953 J/cm3, which is almost 3 times more than that of pristine Ba0.4Sr0.6TiO3 ceramic [15,16,17]. As the BZN content increases, the Pmax declines from 10.92 to 7.03 μC/cm2 and a deep fluctuation from 0.91 to 0.17 μC/cm2 of the Pr, as shown in Table 1. The Pmax sharply decreased as the x content increases, it can be ascribed to the long-range dipole action that was destroyed by the Ti4+ and Nb5+ with different charges at B-site [31], and then the relaxation behavior was enhanced as the BZN value increases. Wst was calculated via polarization–electric filed loops in Fig. 6a, and the Wst has a deep reduction with the x value increases. Meanwhile, the η sharply increases to 92.90% from 77.58% with the increasing BZN value. As shown in Fig. 5, the value of γ increases from 1.56 to 1.76 as the BZN content increases from 0.05 to 0.225 at the temperature range of − 55 °C to 150 °C, indicating the typical transition to relaxor ferroelectric from ferroelectric. So, as the x value increase, the η become larger. This is attributed to the normal ferroelectric has a large hysteresis loss compared with the relaxor ferroelectric, as shown in Fig. 6a.
Figure 6b presents the P-E hysteresis loops of the 0.775BST-0.225BZN sample. A slim P-E hysteresis loop is obtained with the increase of the electric field, indicating the higher efficiency. Table 2 exhibits the energy storage characteristics of the 0.775BST-0.225BZN sample tested in different electric fields. From Fig. 6c and Table 2, it can be found that the Wst increases with the increase of the electric field. Moreover, the η remains as high as 92% with the electric field increases, indicating a relatively high release efficiency of the 0.775BST-0.225BZN ceramic. The Wst is nearly proportional to the square of E. Predictably, the Wst will increase to ~ 1 J/cm3 when the applied electric field is larger than 200 kV/cm due to the large Pmax and low Pr.
The temperature stability of the energy density cannot be ignored due to the harsh working environment of the dielectric capacitor. To further investigate the temperature stability of the energy storage, the hysteresis loops of 0.775BST-0.225BZN ceramic were obtained in the diverse temperatures. Figure 7a, b indicates the energy storage performances of 0.775BST-0.225BZN ceramic in the temperatures range of 25 °C to 150 °C at 10 Hz and 170 kV/cm. A series of slim P-E hysteresis loops were obtained in the tested range of temperatures. Table 3 lists the temperature parameters of the energy storage characteristics. As the temperature increases, it can be observed that the value of Pmax decreases slightly from 7.03 to 5.96 μC/cm2, which indicates the energy storage density has a slight fluctuation in the high-temperature region. The appearance of nonpolar phases may result in a decrease in Pr at high temperatures [34,35,36]. The Wst of the 0.775BST-0.225BZN ceramic in Fig. 7b is smaller than the Wst in Fig. 6a with the temperature increases, this is because the Pmax has a slightly decreased with the same electric fields. From Fig. 7b, it can be found that the Wst of the 0.775BST-0.225BZN ceramic exhibits good temperature stabilize (does not exceed 7%) and the η remains as high as 92% in a very wide range of temperatures from 25 to 150 °C, which suggests good energy storage stabilization. As shown in Fig. 5, the 0.775BST-0.225BZN ceramic meets the X8R specification, indicating the capacitance has good stability in the tested range of temperatures. Besides, the permittivity is proportional to the capacitance. The dielectric constant reflects the polarization behavior of the dielectric indicating the polarization has a good stabilization on temperature. The value of γ is 1.76 as the BZN content is 0.225 and a high diffuse phase transition appears. A high diffuse phase transition means that the reversal of polarization keeps a good stabilization on temperature. So, it is reasonably obtained good temperature stability on energy storage of the 0.775BST-0.225BZN ceramic at the tested range of temperatures. The energy storage performances of the 0.775BST-0.225BZN ceramic compared with other BST-based ceramics are listed in Table 4, suggesting excellent temperature stability of the 0.775BST-0.225BZN ceramic.
The charge–discharge properties of the pulse capacitors can not be ignored in the actual application environment. The discharge current waveforms of the 0.775BST-0.225BZN ceramic were obtained at the diverse electric fields in the underdamped stage as shown in Fig. 8a, which are typical sinusoidal. Figure 8b presents the peak current value, power density (PD = EImax/2S) and current density (CD = Imax/S) of the 0.775BST-0.225BZN ceramic under the diverse electric fields. It can be found that the peak current value increases from 13 to 70 A and the maximum current density up to 357 A/cm2 with the electric field increases to 170 kV/cm. Correspondingly, the 0.775BST-0.225BZN ceramic has a large power density of 30 MW/cm3.
From an application point of view, the good temperature stability of the charge–discharge properties is another important character for the pulse capacitors in the harsh working environment. As shown in Fig. 8c and d, the peak current value, the current density, and the power density of the 0.775BST-0.225BZN ceramic has a slight fluctuation in a wide temperature range of 25 °C to 150 °C. This result can be attributed to the good temperature stability of dielectric properties. The fluctuation of the Imax, CD, PD is less than 3% indicating good temperature stability of the charge–discharge character of the 0.775BST-0.225BZN ceramic. Minor fluctuation in charge–discharge property is beneficial to the 0.775BST-0.225BZN ceramic working in a wide of temperatures.
Figure 9a shows the electric field dependence of overdamped pulsed discharge electric current–time curves at room temperature. The discharge energy density (Wd) of 0.775BST-0.225BZN ceramic was calculated by [7]: \({W}_{d}=R\int {i(t)}^{2}dt/V\), where V and R are the sample volume and total load resistor (205.5Ω), respectively. As shown in Fig. 9b, the Imax and Wd increase from 2.3 to 13.5 A and 0.017 to 0.554 J/cm3 with the electric field increasing from 30 to 170 kV/cm, respectively. It should be noted that the energy density obtained by calculating the integrated area in P-E loops is slightly higher than the calculated by the charge–discharge method. The discrepancy may be attributed to the different impedance and test frequency.
4 Conclusions
A series of (1 − x)BST-xBZN lead-free bulk ceramics were developed via a conventional solid-state synthesis route. The 0.775BST-0.225BZN ceramic was obtained at the sintering temperature as low as 1140 °C. Considering the temperature stabilization of the dielectric performances, the 0.775BST-0.225BZN ceramic meets the X8R specification and its energy storage also shows good temperature stability. Moreover, the 0.775BST-0.225BZN bulk ceramic exhibits a stable dependence of Wst and η on the temperature in a range of 25 °C to 150 °C. The charge–discharge properties also display good temperature stability(variations of the current density and power density are less than 3% over 25–150 °C). The lower sintering temperature and good temperature stability demonstrate that the environment-friendly 0.775BST-0.225BZN bulk ceramic is a potential candidate material for the application of the dielectric capacitor.
References
W. Chen, X. Yao, X. Wei, Relaxor behavior of (Sr, Ba, Bi)TiO3 ferroelectric ceramic. Solid State Commun. 141, 84–88 (2007)
H. Cheng, H. Du, W. Zhou, D. Zhu, F. Luo, B. Xue, Bi(Zn2/3Nb1/3)O3-(K0.5Na0.5)NbO3 high temperature lead-free ferroelectric ceramics with low capacitance variation in a broad temperature usage range. J. Am. Ceram. Soc. 96, 833–837 (2013)
N. Raengthon, T. Sebastian, D. Cumming, I. Reaney, D. Cann, BaTiO3-Bi(Zn1/2Ti1/2)O3-BiScO3 ceramics for high-temperature capacitor applications. J. Am. Ceram. Soc. 95, 3554–3561 (2012)
L. Yang, X. Kong, F. Li et al., Perovskite lead-free dielectrics for energy storage applications. Prog. Mater. Sci. 102, 72–108 (2019)
A. Kusko, J. Dedad, Stored energy - short-term and long-term energy storage methods. IEEE Ind. Appl. Mag. 13, 66–72 (2007)
D. Wang, J. Liu, M. Zeng, C. Zhang et al., Stabilizing temperature-capacitance dependence of (Sr, Pb, Bi)TiO3-Bi4Ti3O12 solutions for energy storage. J. Am. Ceram. Soc. 102, 4029–4037 (2019)
J. Li, J. Liu, M. Zeng et al., High efficiency and power density relaxor ferroelectric Sr0.875Pb0.125TiO3 - Bi(Mg0.5Zr0.5)O3 ceramics for pulsed power capacitors. J. Eur. Ceram. Soc. 40, 2907–2916 (2020)
Q. Zhang, L. Wang, J. Luo, Q. Tang, J. Du, Ba0.4Sr0.6TiO3/MgO composites with enhanced energy storage density and low dielectric loss for solid-state pulse-forming line. Int. J. Appl. Ceram. Technol. 7, 124–128 (2009)
M. Grace, R. Sambasivam, R. Perumal, V. Athikesavan, Enhanced synthesis, structure, and ferroelectric properties of Nb-modified 1–x[Bi0.5(Na0.4K0.1)(Ti1−xNbx)]O3−x(Ba0.7Sr0.3)TiO3 ceramics for energy storage applications. J. Aust. Ceram. Soc. 56, 157–165 (2020)
Y. Huang, Y. Wu, W. Qiu, J. Li, X. Chen, Enhanced energy storage density of Ba0.4Sr0.6TiO3–MgO composite prepared by spark plasma sintering. J. Eur. Ceram. Soc. 35, 1469–1476 (2015)
Y. Wang, Y. Pu, Y. Cui, Y. Shi, H. Zheng, Enhanced energy storage density of Ba0.4Sr0.6TiO3 ceramics with additive of Bi2O3-B2O3-ZnO glass. Mater. Lett. 201, 203–206 (2017)
J. Zhang, J. Zhai, H. Jiang, X. Yao, Raman and dielectric study of Ba0.4Sr0.6TiO3-MgAl2O4 tunable microwave composite. J. Appl. Phys. 104, 567–572 (2008)
Z. Song, H. Liu, Z. Wang et al., Effect of grain size on energy storage properties of (Ba0.4Sr0.6)TiO3 paraelectric ceramics. J. Eur. Ceram. Soc. 34, 1209–1217 (2014)
B. Parija, T. Badapanda, S.K. Rout et al., Morphotropic phase boundary and electrical properties of 1–x[Bi0.5Na0.5]TiO3–xBa[Zr0.25Ti0.75]O3 lead-free piezoelectric ceramics. Ceram. Int. 39, 4877–4886 (2013)
Y. Wang, Z. Shen, Y. Li et al., Optimization of energy storage density and efficiency in BaxSr1-xTiO3 (x≤0.4) paraelectric ceramics. Ceram. Int. 41, 8252–8256 (2015)
Z. Zheng, Y. Zhang, X. Wang, Q. Zhang, I. Baturin, Variation of DC breakdown strength with phase transition temperature in (Ba1-xSrx)TiO3 ceramics. Ferroelectrics 442, 115–122 (2013)
Z. Song, H. Liu, M.T. Lanagan et al., Thermal annealing effects on the energy storage properties of BST ceramics. J. Am. Ceram. Soc. 100, 3550–3557 (2017)
M. Zeng, J. Liu, H. Yu et al., NiNb2O6-BaTiO3 ceramics for energy-storage capacitors. Energy Technol. 6, 899–905 (2018)
C. Diao, H. Liu, H. Hao, M. Cao, Z. Yao, Effect of SiO2 additive on dielectric response and energy storage performance of Ba0.4Sr0.6TiO3 ceramics. Ceram. Int. 42, 12639–12643 (2016)
W. Li, D. Zhou, L. Pang, Enhanced energy storage density by inducing defect dipoles in lead free relaxor ferroelectric BaTiO3-based ceramics. Appl. Phys. Lett. 110, 1329021–1329025 (2017)
L. Wu, X. Wang, L. Li, Lead-free BaTiO3–Bi(Zn2/3Nb1/3)O3 weakly coupled relaxor ferroelectric materials for energy storage. RSC Adv. 6, 14273–14282 (2016)
L. Zhang, L.X. Pang, W.B. Li, D. Zhou, Extreme high energy storage efficiency in perovskite structured (1–x) (Ba0.8Sr0.2)TiO3 - xBi(Zn2/3Nb1/3)O3 (0.04≤x≤0.16) ceramics. J. Eur. Ceram. Soc. 40, 3343–3347 (2020)
F. Li, J. Zhai, B. Shen et al., Influence of structural evolution on energy storage properties in Bi0.5Na0.5TiO3-SrTiO3-NaNbO3 lead-free ferroelectric ceramics. J. Appl. Phys. 121, 054103 (2017)
P.S. Dobal, A. Dixit, R.S. Katiyar et al., Micro-Raman study of Ba1-xSrxTiO3 ceramics. J. Raman Spectrosc. 32, 147–149 (2001)
Z. Shen, X. Wang, B. Luo, L. Li, BaTiO3-BiYbO3 perovskite materials for energy storage applications. J. Mater. Chem. A 3, 18146–18153 (2015)
D. Zheng, R. Zuo, D. Zhang et al., Novel BiFeO3-BaTiO3-Ba(Mg1/3Nb2/3)O3 lead-free relaxor ferroelectric ceramics for energy-storage capacitors. J. Am. Ceram. Soc. 98, 2692–2695 (2015)
K. Wang, A. Hussain, W. Jo, J. Rödel, Temperature-dependent properties of (Bi1/2Na1/2)TiO3-(Bi1/2K1/2)TiO3-SrTiO3 lead-free piezoceramics. J. Am. Ceram. Soc. 95, 2241–2247 (2012)
H.B. Yang, F. Yan, Y. Lin, T. Wang, Improvement of dielectric and energy storage properties in SrTiO3-based lead-free ceramics. J. Alloys Compd. 728, 780–787 (2017)
K.N. Singh, P.K. Bajpai, Dielectric relaxation in pure columbite phase of SrNb2O6 ceramic material: impedance analysis. WJCMP 1, 37–48 (2011)
T. Shi, L. Xie, L. Gu, J. Zhu, Why Sn doping significantly enhances the dielectric properties of Ba(Ti1-xSnx)O3. Sci. Rep. 5, 8606 (2015)
X. Jiang, H. Hao, S. Zhang et al., Enhanced energy storage and fast discharge properties of BaTiO3 based ceramics modified by Bi(Mg1/2Zr1/2)O3. J. Eur. Ceram. Soc. 39, 1103–1109 (2019)
F. Li, M. Zhou, J. Zhai, B. Shen, H. Zeng, Novel barium titanate based ferroelectric relaxor ceramics with superior charge-discharge performance. J. Eur. Ceram. Soc. 38, 4646–4652 (2018)
M. Zhou, R. Liang, Z. Zhou, X. Dong, Novel BaTiO3-based lead-free ceramic capacitors featuring high energy storage density, high power density, and excellent stability. J. Mater. Chem. C 6, 8528–8537 (2018)
X.Y. Ye, Y.M. Li, J.J. Bian, Dielectric and energy storage properties of Mn-doped Ba0.3Sr0.475La0.12Ce0.03TiO3 dielectric ceramics. J. Eur. Ceram. Soc. 37, 107–114 (2017)
F. Li, K. Yang, X. Liu et al., Temperature induced high charge-discharge performances in lead-free Bi0.5Na0.5TiO3-based ergodic relaxor ferroelectric ceramics. Scr. Mater. 141, 15–19 (2017)
G. Viola, R. McKinnon, V. Koval, A. Adomkevicius, S. Dunn, H. Yan, Lithium-induced phase transitions in lead-free Bi0.5Na0.5TiO3 based ceramics. J. Phys. Chem. C 118, 8564–8570 (2014)
P. Ren, Q. Wang, S. Li, G. Zhao, Energy storage density and tunable dielectric properties of BaTi0.85Sn0.15O3/MgO composite ceramics prepared by SPS. J. Eur. Ceram. Soc. 37, 1501–1507 (2017)
Acknowledgements
The author would like to thank the State Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China (Chengdu, Sichuan, 610054, People’s of China) for their help in dielectric properties testing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Z., Li, H., Qing, Z. et al. Temperature stability of lead-free BST-BZN relaxor ferroelectric ceramics for energy storage capacitors. J Mater Sci: Mater Electron 32, 752–763 (2021). https://doi.org/10.1007/s10854-020-04854-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04854-x