Abstract
This work was aimed to study the influence of nitrogen reactive gas and substrate temperature, on the crystalline structure, morphology, sheet resistance and optical properties of titanium nitride thin films. The samples were deposited on glass substrates by the direct current reactive magnetron sputtering method and were controlled to have thicknesses of 150 nanometer (nm). The crystal structures of the samples were checked using the X-ray diffraction technique. The average crystallite size was calculated by using the Scherrer equation. Atomic Force Microscopy was performed on all samples to investigate any change in the crystallite sizes, morphology and also in the roughness of the film’s surface. With the variation in substrate temperature, the optical properties (refractive index, optical band gap and extinction coefficient) of films also changed. The experimental reflectance and absorption spectra of the samples have been characterized by spectrophotometer measurements. Optical band-gap values decrease from 4.01 eV at 300 °C to 3.59 eV at 500 °C. Optical refractive index (n) and extinction coefficient (k) are strongly dependent on the flow rate of nitrogen and substrate temperature. Furthermore, the sheet resistance of the TiN samples are dependent on the investigated deposition parameters. The high reflectance, good morphology, improved crystallinity and low sheet resistance maintained by the films, made them very favorable to be used in both optical and electronic applications, and also as protective and decorative coatings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last few decades, titanium nitride (TiN) thin films have attracted considerable interest with widespread applications in several fields [1]. Most of the researches concerning TiN films focused on several interesting properties such as hardness, high thermal and chemical stability; low electrical and thermal resistivity; and bright golden color [2].
Due to their hardness, wear resistance and thermal properties, the TiN films have been very favorable in surface engineering as protective coatings on high speed tools [1]. Recently, TiN films have been used as electrodes in microelectronic devices, due to their thermal stability and low electrical resistivity [3]. However, it was reported that the electrical resistivity of TiN depends strongly on its stoichiometry and morphology [4]. Many researchers have also reported that TiN films are widely used as micro-electrical contacts and resistors, also used on pens, watches and others goods due to their special shiny golden color [5, 6]. In particular, thin TiN films in the thickness range (20–80 nm) were also proved to be suitable as selectively transmitting heat mirrors [7].
In addition to its electronic properties, TiN is appealing for electronic devices due to its compatibility with complementary metal oxide silicon (CMOS) technology, caused by its high electron mobility and refractory character [1].
More recently, titanium nitride coatings have been applied in areas such as abrasion resistant coatings on tool steels, decorative coatings in architecture, non-toxic exterior for medical implants, diffusion barrier layers in semiconductor devices and flat panel displays [6, 7].
These features were found to depend strongly on crystallographic orientation and crystallite size of the deposited films [3, 4]. It is well-known that, the properties of TiN depend on the processing method used and deposition parameters, particularly, the nitrogen gas pressure and the substrate temperature [2].
TiN films have been prepared using different methods such as sputtering [8,9,10,11,12], chemical vapour deposition [13], electron-beam evaporation [14], vacuum arc evaporation [15, 16] and pulsed laser deposition [17].
Among these processes, sputtering is particularly suitable for deposition of TiN films due to the ease of adjusting the deposition parameters which play an important role in the stoichiometry and morphology of the deposited films. Moreover, the film deposition parameters can be adjusted with high precision to give high quality uniform and dense thin films.
In fact, the deposition of TiN thin films was well described in the work of many researchers in the last two decades due to the various applications of such conducting nitride, and the necessity to explore their performance under different conditions in the fields of electronics, optics, wear resistance in automobile industries, steel cutting tools and biomedical materials [2, 6, 10].
In the present work, we have focused on the effect of substrate temperature and reactive gas pressure on the properties of TiN films. Therefore, TiN films were deposited by a reactive sputtering technique, using a titanium target in a mixture of argon-nitrogen atmosphere. In order to improve properties of TiN films, the ratio of argon: nitrogen gas pressure was changed to investigate its effects on the film properties, also the substrate temperatures. Our focus was on the changes in the morphology of films deposited on glass substrate, their crystalline structure; electrical, spectral and optical properties and to investigate the possibility of using such films as optical, decorative and protective coatings. Each characterization on the TiN samples was repeated three times in the same conditions, while the average value of the obtained results was used in all illustrations to maintain the accuracy and consistency of our results.
2 Experimental details
2.1 Substrate cleaning procedure
Prior to titanium nitride thin film deposition, soda-lime glass (25 × 25 mm2) substrates were cleaned by sonication with detergent (acetone) for 15 min, rinsed with deionized water for 5 min, dried in the oven at 110 °C for outgassing. The substrates and acetone were purchased from Sigma-Aldrich.
2.2 Thin film deposition
Titanium nitride thin films were deposited with an Oxford plasma 400 DC magnetron sputtering system, using a circular 50.8 mm diameter and 6.35 mm thick Titanium target of 99.998% purity supplied by K.J. Lesker, UK.
The titanium nitride TiN thin films (140–150 nm thick) were sputtered onto clean –glass substrate and under different N2 flow rates 2–4–6 standard cubic centimeters (sccm). Pure argon gas (Ar, 99.999%) and nitrogen gas (N2, 99.999%) were used as sputter and reactive gases, respectively. The TiN samples were prepared by a vacuum base pressure at 4 × 10− 4 Pa. The deposition time was adjusted to have an average thickness of 150 nm for all samples. Pre-sputtering for 1 min was performed in an argon atmosphere to ensure that the titanium target is clean and free of any residual which affect the quality of the TiN films. To assess the uniformity of the deposited TiN films, the substrate was rotated with a speed of 80 rpm.
The Argon–nitrogen (Ar:N2) concentration ratio was changed from 10:2 to 10: 4 sccm and then to 10:6 sccm (as mentioned in Table 1) to investigate its effects on the optical, structural and morphological properties. In addition, various substrate temperatures were used to assess its influence on the film properties, while the other deposition parameters such as Substrate to target distance and Cathode/target power were kept constant.
2.3 Atomic force microscopy
The surface morphology and roughness of the films before and after annealing were observed using Atomic Force microscopy (AFM) in contact mode. The structural properties and crystallite sizes of the grown films were investigated using an Atomic Force Microscopy AFM (Multi-Mode 8-Bruker).
2.4 Spectral characterization
A spectrophotometer apparatus (Perkin Elmer Lambda 950 UV–VIS) was used for measuring the spectral properties such as reflectance, transmission and absorption of the thin films in the visible region (380–780 nm) deposited on glass substrates. Glass substrate was used as reference to calibrate the apparatus.
2.5 X-ray diffraction
XRD was used to study the effect of Argon-nitrogen gas concentration and substrate temperatures on the crystal structure of the TiN films. A Bruker-AXS D8 ADVANCE X-ray diffractometer with Cukα1 Copper kα X-rays of wavelength 1.54 Å were used for the diffraction.
2.6 Sheet resistance
Sheet resistance of the TiN films was measured using a 4 point probe instrument (JANDEL, Model RM3000).
2.7 Ellipsometer measurement
The optical constants such as refractive index (n) and extinction coefficient (k) for each sample were measured using an Ellipsometer Spectroscopy (ES) (UVISEL, from HORIBA).
3 Results and discussions
3.1 XRD results of TiN thin films
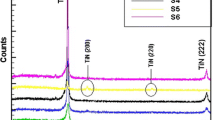
The X-ray diffraction patterns of TiN films grown at different substrate temperatures on glass substrates (Ts= 300 °C, 400 °C, 500 °C) are shown in Fig. 1. For the substrate temperatures (300 °C and 400 °C), only (111) peak appeared at 2θ = 38.31°. Increasing the substrate temperature to 500 °C resulted in an increase of the (111) peak’s intensity and the appearance of another peak (222) at 2θ = 78.38°. This may be explained that excess substrate temperature during the deposition process, has a good impact on the reactive deposition process and the crystalline structure.
The intensity of TiN (111) diffraction peak was the strongest, and no change to the peak’s positions for the three different temperatures used with an argon: nitrogen gas of concentration (10:2 sccm).
The crystallite size of the TiN was then calculated using the following Scherrer expression [12]:
where, D is the crystallite size, λ is the X-ray wavelength (Cu-Kα, 0.154 nm); θ is the diffraction angle and β is the line broadening at half the maximum intensity (FWHM). This quantity (β) is sometimes denoted as Δ(2θ). The broadening of the (111) and (222) was used to calculate the average crystallite size using Scherrer’s equation. Each peak of the diffraction pattern has a broadening. The full width at half maximum of each peak is the measurements of this broadening.
Figure 2 shows the relation between the lattice constant and crystallite size with substrate temperature, the crystallite size has increased with the increase of substrate temperature from nearly 28 to 39 nm for 300 °C and 400 °C respectively, and then followed by a large increase again to 96 nm at 500 °C. The high value of crystallite size of the TiN films grown at 500 °C is also an indication of its microstructure densification, and this could be confirmed by the higher intensity of the (111) diffraction peak at this temperature. Increasing the substrate temperature also increased the grouping and the nucleation of Ti and N2 atoms making the TiN films and resulted in an increase of the crystalline size [3].
Thus, the results clearly demonstrate that the crystallite size of the TiN films deposited at Ar:N2 (10:2 sccm) flow rate and different substrate temperatures increases with increasing temperature. Similar results for sputtered TiN films reported that the crystallite size increases with increasing of substrate temperature [4, 7].
Meanwhile, a similar behavior was observed for the lattice constants of the TiN films??. At 300 °C, the lattice constant was 4. , followed by a slight increase to 4.245 at 400 °C and 4.254 at 500 °C, respectively.
The lattice parameter of TiN films deposited at Ar:N2 ratio of 10:2 sccm is found to be close to the value of the bulk lattice parameter (a = 4.24 Å) and it possibly indicates the formation of stoichiometric TiN.
Many researchers have reported that the increase of substrate temperature during film deposition improves the physical properties of films by modifying the crystallite size and altering the optical band gap of the sputtered TiN films from 4.06 to 3.4 eV [14].
The substrate temperature has also a strong impact on the TiN film’s microstructure which resulted in dense and stoichiometric TiN with the absence of voids and small crystallite size.
3.2 Morphology of TiN thin films
Atomic Force Microscopy (AFM) was used to find surface roughness, crystallite sizes and morphology of the TiN films deposited at various substrate temperatures. Figure 3a–c shows the AFM images for films deposited at 300 °C, 400 °C and 500 °C, respectively. Table 2 represents the average roughness (Ra) of the TiN films measured by AFM. As shown in Table 2, the roughness decreased from 3.64 nm for samples deposited at 300 °C to ~ 3.32 nm on average, and then the surface roughness increased highly to 6.87 nm at a N2 flow rate of 2sccm. Figure 3d–f shows the morphology images of the TiN thin films deposited at 300 °C with N2 flow rate of 2, 4 and 6 sccm, respectively. A summary of the average surface roughness Ra for TiN films is included in Table 2. Figure 4 shows AFM 3D images of surface roughness for TiN films at various substrate temperatures. (10 µm). Figure 4a–c for various nitrogen flow (2–4–6 sccm) at 300 °C and Fig. 4d–f for various substrate temperature (300–400–500 °C) at 2 sccm Nitrogen gas flow.
An average roughness of 3.64 nm was obtained at 2sccm of N2, followed by a reduction to 2.92 nm at 4sccm of N2, and then reduced to nearly 0.801 nm. By comparing these results with the preferable orientation discussed previously, we concluded that when the orientation is in the direction of (111) the surface is smoother than the (222) directions. Similarly, researchers reported that dense and uniform TiN films can be obtained by varying the N2 flow or substrate temperatures such as 200 °C, 400 °C and even 600 °C, corresponding to sharp XRD peaks of crystalline structure at (111) and (222) [15,16,17].
3.3 Effects of substrate temperature on the optical properties of TiN films
The reflectance of protective coatings is a critical factor for optical and decorative applications. Therefore, we have characterized the reflectance of the TiN thin films grown at different substrate temperatures (300 °C, 400 °C, 500 °C) in the range of 300–2000 nm and for various Argon: Nitrogen (Ar:N2) gas flow rate (10:2, 10:4, 10:6 sccm). The reflectance spectra of all the TiN films are shown in Fig. 5. The optical reflectance of the three films slightly decreased in the visible region with the increase of their substrate temperatures during the growth process from 99.1% at 300 °C to 98.2% at 500 °C. We could explain this result from the fact that all the films have a fine structure composed by grains having only small sizes, as previously shown by AFM characterizations in Figs. 3 and 4. We relate this behavior to an improvement of the layer quality with the temperature during growth, as already observed by the work done in [18] who showed the same trend in their TiN thin films when deposited at a temperature varying from 300 to 500 °C. Thereby, the low absorbance and high reflectance results obtained, confirm that the so-produced TiN thin films could be used as optical and protective coatings.
For simplicity, the reflectance values at the visible (550 nm), infrared (700 nm) and at (2000 nm) regions versus variable substrate temperatures and gas flow rates, are shown in Tables 3 and 4, respectively.
3.4 Optical band gap of TiN films
Energy gap is determined using MOH and Davis’ model [19] and for direct band gap the following:
where A is a constant, α is the absorption coefficient, h is the Plank’s constant, and ν is the photon’s frequency.
To determine the direct band gap of the sputtered TiN films, the variations of (αhν)2 versus (hν) were plotted for the various Nitrogen flow rates as shown in Fig. 6. The optical band gaps increased from 3.72 eV at 20% to 3.96 eV at 40%, then decreased to 3.88 eV at 60% N2 flow rate. A summary of the optical band gap is shown in Table 5. This decrease in the band gap may be attributed to the increment in the grain sizes of films as a result of increasing the substrate temperature as previously shown in (Fig. 2). This is in agreement with the results reported recently by researchers on the effects of substrate temperature on the properties of sputtered TiN films [20].
Figure 7 represents the variation of energy gap (Eg) and reflectance with N2 flow rate. As shown in the figure, the value of Eg increases from 3.72 to 3.95 eV and a N2 flow rate at 20% and 40%, respectively. While, the Eg deceased to 3.89 eV at a N2 flow rate of 60%.
Similarly, as indicated by different authors, the increase in N2 flow rate during the??TiN resulted in the improvement of films structure and the reduction in Eg [21].
Meanwhile, the reflectance of TiN films remained constant at 98.3% for all N2 gas flow rates used.
Figure 8 shows the variation of energy gap (Eg) and reflectance (R) with substrate temperature. It can be seen from Fig. 8 that increasing the substrate temperature resulted in a reduction of the optical band gap values, 4.0, 3.83 and 3.59 eV for 300, 400 and 500 °C, respectively. Such reduction of optical band gap can be attributed to the increment in the crystallite sizes of TiN films as mentioned previously and can be seen in Fig. 2. An optical band gap of 3.43 eV was reported for sputtered TiN at substrate temperatures of 300 °C, 400 °C [20], while for TiN films, the value of Eg was 4.06 eV for samples deposited at room temperature.
A similar behavior was observed for the reflectance which was slightly reduced from 98.6 to 98.34%.
3.5 Electrical properties of the TiN films
Sheet resistance of TiN film was measured using the four-point probe method. The average sheet resistance of each TiN film was measured from three different positions of the film’s surface.
The variation in sheet resistance Rs as a function of nitrogen flow rate (20%, 40% and 60%) during TiN film deposition at a 300 °C substrate temperature is shown in Fig. 9. The sheet resistance (76 Ω−2) of the deposited samples is the lowest when the nitrogen flow rate is lowest (20%). Sheet resistance increased with increasing nitrogen content during TiN deposition while maintaining constant temperature and reaches the maximum value of 127 Ω−2 at 40% N2 rate, then maintained the same resistance value for the higher nitrogen flow rate of 60%.
As the working gas pressure increases, the sheet resistance increased linearly. High working pressure resulted in smaller grain size and higher packing density of films. Consequently, the sheet resistance of TiN film increased.
From Fig. 10 which represents the sheet resistance of TiN film as a function of substrate temperature at an Argon: Nitrogen (10:2sccm) flow rate, the lowest value (4.53 Ω−2) was obtained at 500 °C and the highest value (128. 72 Ω−2) at 400 °C. While the value of (76. 33 Ω−2) was obtained at 300 °C. This indicates that increasing the substrate temperature showed some advantages and some disadvantages at the same time. The primary reason to decrease sheet resistance contributed to the smoothness of surface of TiN film as the substrate temperature increased. Here, we could conclude that the 500 °C temperature is more suitable to obtain high quality films for the Argon:Nitrogen gas flow rate (10:2 sccm). This is mainly due to the presence of Ti ion or N ion in the TiN film resulting from the reactive deposition process which promotes conducting process easily [22,23,24]. Further investigation of the optimum temperature and nitrogen flow rate is necessary to provide TiN films with low sheet resistance.
3.6 Ellipsometric measurements of TiN thin films
In order to extract the optical constant n and k, the experimental spectra have been fitted by the layer model. For simplicity, the optical constants n and k of the TiN films deposited for various Ar:N2 gas concentration and for various substrate temperatures are shown in Figs. 11 and 12, respectively. To obtain the optical constants (n) and (k) of deposited TiN films, we used a combination of Lorentz oscillator and Drude oscillator and measured for the glass substrates to find the refractive index n. The fitting was performed by minimizing the mean square error and the backside substrate reflection was also taken into account. The thickness and the optical constants n and k of TiN films were extracted based on the best fitting between the simulated spectra and the experimental data. Good agreement (not shown here) has been obtained between the measured thickness and the values determined by Ellipsometry.
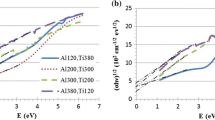
As can be seen from Fig. 11 which represents the variation of optical constants n and k with Ar: N2 gas concentration versus wavelength, the refractive index n at a wavelength of 1200 nm, had a slight decrease in value, with an increase of nitrogen gas flow rate. In contrast to the behavior of n, the extinction coefficient k increased as the nitrogen gas concentration increased. Similar behavior of both n and k was also observed at a wavelength of 2000 nm.
In the visible region, the refractive index of TiN films is around 4.76, which is similar to previous optical measurements performed on sputtered TiN films [5, 25].
Figure 12 shows the variation of optical constants n and k with substrate temperature versus wavelength. For the refractive index n at 1200 nm wavelength, the n values were varied from 7.9 to 300 °C to 6.8 at 500 °C. There is also a small variation in the extinction coefficient with substrate temperatures at 1200 nm, as it varies from 0.71 to 500 °C to 1.25 at 300 °C, whereas at 2000 nm the variation spans from 1.1 to 2 for the same substrate temperatures. In recent work, Banerjee and co-authors reported on the optical properties of TiN films at a wavelength of 2000 nm with extinction coefficient and refractive index of 4.6 and 5.9, respectively [26]. Furthermore, Banerjee and co-authors reported an extinction coefficient k of 4.48 for (100 nm thick) polycrystalline sputtered TiN films at 1550 nm wavelength [27].
4 Conclusions
The TiN films were prepared by d.c. sputtering magnetron sputtering with substrate temperatures from 300 °C, 400 °C and 500 °C and various nitrogen gas pressure from 2, 4 and 6 sccm.
With the increase of the substrate temperature, the microstructure of the thin films improved with the XRD diffractogram revealing that the TiN films deposited at 500 °C possessed good crystalline face-centered cubic (FCC) structure, with a preferred plane orientation along (111). The diffraction peaks of the film become more intense when the substrate temperature increases, which in turn leads to the increase in the crystallite size as well as the enhancement of crystallinity, and the optical band gap changed from 3.99 eV at 300 °C to 3.76 at 500 °C. The maximum value of crystallite size of the TiN film deposited at 500 °C was confirmed by the higher intensity of the diffraction peaks (111) and (222) at this temperature. It is also evident from the AFM micrographs that the shape of the crystallites does not change with increasing temperature. The TiN films showed a slight increase of reflection with the increase of substrate temperature.
The deposition of TiN by sputtering at various nitrogen–argon concentrations gives high quality films with dense and smoother surface. The lattice parameter of TiN films deposited at (Ar:N2 )ratio of (10:2) sccm was found to be close to the value of the bulk lattice parameter (a = 4.24 Å) and it possibly indicates the formation of stoichiometric TiN. Increasing the nitrogen gas ratio resulted in a reduction of the average roughness of the TiN films. Sheet resistance of 4.53 Ω−2 at a temperature of 500 °C for the (Ar:N2 ) gas ratio of (10:2) sccm. In the visible region, the optical refractive index of films was 4.76, close to similar results reported earlier by other researchers.
All TiN films showed a high average reflectance above 98% and a very low absorbance less than 2%. The reflectance characteristics of films on glass showed a typical spectral selectivity. The small crystallite sizes, fine crystalline structure and better spectra selectivity is a measurement of the quality and potential use of TiN for optical applications. The golden-yellow color of the TiN films highlights the importance of its use for protective and decorative applications. It is clear the deposition parameters can be adjusted to improve the TiN thin film properties by choosing an optimum substrate temperature and Ar:N2 gas ratio to match the actual gold color required for decorative applications.
References
P. Patsalas, N. Kalfagiannis, S. Kassavetis, Optical properties and plasmonic performance of titanium nitride. Materials (Basel) 8(6), 3128–3154 (2015)
M. Birkholz, K.-E. Ehwald, D. Wolansky, I. Costina, C. Baristiran-Kaynak, M. Fröhlich, H. Beyer, A. Kapp, F. Lisdat, Corrosion-resistant metal layers from a CMOS process for bioelectronics applications. Surf. Coat. Technol. 204(12–13), 2055–2059 (2010)
P. Patsalas, N. Kalfagiannis, S. Kassavetisa, G. Abadias, D.V. Bellasd, Ch. Lekka, E. Lidorikis, Conductive nitrides: growth principles, optical and electronic properties, and their perspectives in photonics and plasmonics. Mater. Sci. Eng. R 123, 1–55 (2018)
A. Kavitha, R. Kannan., PSReddy;S. Rajashabala, The effect of annealing on the structural, optical and electrical properties of Titanium Nitride (TiN) thin films prepared by DC magnetron sputtering with supported discharge. J. Mater. Sci. 27, 10427–10434 (2016)
M.N. Gadalla, K. Chaudhary, C.M. Zgrabik, F. Capasso, E.L. Hu, Imaging of surface plasmon polaritons in low-loss highly metallic titanium nitride thin films in visible and infrared regimes. Opt. Express 28(10), 14536 (2020). https://doi.org/10.1364/OE.391482
B. Wei, H. Liang, D. Zhang, Z. Qi., H. Shen, Z. Wang, Magnetron sputtered TiN thin films toward enhanced performance supercapacitor electrodes. Mater. Renew Sustain. Energy 7, 11 (2018). https://doi.org/10.1007/s40243-018-0117-9
N. Ghobadi, M. Ganji, C. Luna, A. Arman, A. Ahmadpourian, Effects of substrate temperature on the properties of sputtered TiN thin films. J. Mater. Sci. 27, 2800–2808 (2016). https://doi.org/10.1007/s10854-015-4093-x
M.A. Domínguez-Crespo, A.M. Torres-Huerta, E. Rodríguez, A. González-Hernández, S.B. Brachetti-Sibaja, H.J. Dorantes-Rosales, A.B. López-Oyama, Effect of deposition parameters on structural, mechanical and electrochemical properties in Ti/TiN thin films on AISI 316L substrates produced by R. F. Magnetron sputtering. J. Alloys Compd. 746(25), 688–698 (2018)
H.A. Abd El-Fattah, I.S. El-Mahallawi, M.H. Shazly, W.A. Khalifa, Optical properties and microstructure of TiNxOy and TiN thin films before and after annealing at different conditions. Coatings 9(1), 22 (2019). https://doi.org/10.3390/coatings9010022
H. Liang, J. Xu, D. Zhou, X. Sun, Y. Bai, Thickness dependent microstructural and electrical properties of TiN thin films prepared by DC reactive magnetron sputtering. Ceram. Int. 42, 2642–2647 (2016)
T. Zhou, D. Liu, Y. Zhang, T. Ouyang, J. Suo, Microstructure and hydrogen impermeability of titanium nitride thin films deposited by direct current reactive magnetron sputtering. J. Alloys Compd. 688, 44–50 (2016)
A. Baptista, F. Silva, J. Porteiro, J. Míguez, G. Pinto, Sputtering physical vapour deposition (PVD) coatings: a critical review on process improvement and market trend demands. Coatings 8(11), 402 (2018). https://doi.org/10.3390/coatings8110402
L.V. Fieandt., T. Larsson, E. Lindahl., O. Bäcke., Chemical vapor deposition of TiN on transition metal substrates. Surf. Coat. Technol. 334, 373–383 (2018)
T. Ryynänen, M. Toivanen, T. Salminen, L. Ylä-Outinen, S. Narkilahti, J. Lekkala, Ion beam assisted e-beam deposited TiN microelectrodes—applied to neuronal cell culture medium evaluation. Front. Neurosci. (2018). https://doi.org/10.3389/fnins.2018.00882
O. Singh, R.P. Dahiya, H.K. Malik, P. Kumar, V. Singh, Investigation of titanium nitride thin films treated in hot cathode arc discharge plasma system. Appl. Sci. Lett. 2(1), 37–41 (2016). https://doi.org/10.17571/appslett.2016.02006
Y Ma, J Yang, X Tian, C Gong, W Zheng, Y He, Z Gao (2020) Microstructure, adhesion, mechanical and corrosion properties of TiN coatings deposited by high energy pulse-enhanced vacuum arc evaporation. J. Adhes. Sci. Technol. 34(10):1040–1061 (2020). https://doi.org/10.1080/01694243.2019.1690774
R.P. Sugavaneshwar, S. Ishii, T.D. Dao, A. Ohi, T. Nabatame, T. Nagao, Fabrication of highly metallic TiN films by pulsed laser deposition method for plasmonic applications. ACS Photon. 5, 814–819 (2018)
N.K. Ponon, D.J.R. Appleby, E. Arac, P.J. King, S. Ganti, K.S.K. Kwa, A. O’Neill. Effect of deposition conditions and post deposition anneal on reactively sputtered titanium nitride thin films. Thin Solid Films 578, 31–37 (2015)
V. Chawla, J. Rengaswamy, R. Chandra, Structural characterizations of magnetron sputtered nanocrystalline TiN thin films. Mater. Charact. 59, 1015–1020 (2008)
A. Albert Irudayaraj, R. Srinivasan, E. Mohandas, K. Parasuraman, S. Kalainathan, K. Ramachandran, Photoacoustic measurement of thermal properties of TiN thin films. J. Mater. Sci. 43, 1114–1120 (2008)
P. Patsalas, C.A. Charitidis, S. Logothetidis, The effect of substrate temperature and biasing on the mechanical properties and structure of sputtered titanium nitride thin films. Surf. Coat. Technol. 125(1), 335–340 (2000). https://doi.org/10.1016/S0257-8972(99)00606-4
Y.L. Jeyachandran, S.K. Narayandass, D. Mangalaraj, S. Areva, J.A. Mielczarski, Properties of titanium nitride films prepared by direct current magnetron sputtering. Mater. Sci. Eng. A 445–446, 223–236 (2007)
J.-H. Huang, K.-W. Lau, G.-P. Yu, Effect of nitrogen flow rate on structure and properties of nanocrystalline TiN thin films produced by unbalanced magnetron sputtering. Surf. Coat. Technol. 191(1), 17–24 (2005)
E. Penilla, J. Wang, Pressure and temperature effects on stoichiometry and microstructure of nitrogen-rich TiN thin films synthesized via reactive magnetron DC-sputtering. J Nanomater (2008). https://doi.org/10.1155/2008/267161
M. Nader-Ghobadi, C. Gauji, A. Luna, A. Arman, A. Ahmadpourian, Effects of substrate temperature on the properties of sputtered TiN thin films. J. Mater. Sci. 27(3), 2800–2808 (2015)
A. Banerjee, R.M. Heath, D. Morozov, D. Hemakumara, U. Nasti, I. Thayne, H.R. Hadfield. Optical properties of refractory metal based thin films. Opt. Mater. Express 8(8), 2072–2089 (2018)
M. Dai, W. Guo, X. Liu, M. Zhang, Y. Wang, L.F. Wei, G.C. Hilton, J. Hubmayr, J. Ullom, J. Gao, M.R. Vissers. Measurement of optical constants of TiN and TiN/Ti/TiN multilayer films for microwave kinetic inductance photon-number-resolving detectors. J. Low Temp. Phys. 194(9), 2095–2099 (2019)
Acknowledgements
The researchers acknowledge the Deanship of Scientific Research at Al Imam Mohammad Ibn Saud Islamic University, Saudi Arabia, for finance this project under the grant no. (381214). We also thank Mr. Fahad S. Alghamdi at the National Nanotechnolology Center, King Abdul-Aziz City for Science and Technology for the technical support and his help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mustapha, N., Fekkai, Z. Impact of nitrogen reactive gas and substrate temperature on the optical, electrical and structural properties of sputtered TiN thin films. J Mater Sci: Mater Electron 31, 20009–20021 (2020). https://doi.org/10.1007/s10854-020-04523-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04523-z