Abstract
Nonlinear optical single crystals of pure l-Alanine (LA) and Zirconium Nitrate-doped l-Alanine (ZNLA) were grown by low-temperature solution growth slow evaporation technique at room temperature. Single crystal and powder XRD confirms the orthorhombic structure. The average crystallite size and lattice strain are 53.21 nm and 0.0048 for LA and 62.17 nm and 0.0041 for ZNLA. FTIR reveals the presence of functional groups in the grown crystal. The optical analysis confirms the high transparency, the obtained direct and indirect transition bandgap of LA and ZNLA are 5.4 eV, 4.84 eV and 5.32 eV, 4.11 eV. The dielectric constant and dielectric loss of the gown materials are low at high frequencies. The relative second harmonic efficiency of l-Alanine and Zirconium Nitrate-doped l-Alanine are 1.15 and 1.47 times higher than that of KDP. The magnetic parameters were identified with VSM analysis. The thermal stability of LA and ZNLA single crystals was found stable up to 193.5 35 °C and 248.35 °C for ZNLA. Photoconductivity reveals that both the material LA and ZNLA have negative photoconductivity nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nonlinear optical single gems have been playing a critical part in numerous logical and industrials applications such as laser, optical information storage devices, optical switching, optical computing, optical communication, optoelectronics, optical modulating, optical limiting, and signal transmission [1,2,3,4,5,6]. Organic nonlinear optical materials have drawn different fields of research due to high thresholds for laser damage and optical propagation, broad third-order nonlinear optical sensitivity and nonlinear coefficient, strong chemical stability, etc. [6, 7]. Amino acid crystals are becoming more and more essential owing to excessive second harmonic nonlinear optical materials [8,9,10]. To avoid these limitations, researchers have focused on introducing new types of highly optical, thermal, and mechanical properties of single crystals by doping inorganic materials that generate organic–inorganic complexes [11]. Due to excellent nonlinear optical nature, researchers have reported works on l-Alanine such as l-Alanine Tartrate Single Crystals [12]; Optical, thermal, and mechanical studies on nonlinear optical material diglycine barium chloride monohydrate (DGBCM) single crystal [13]; Growth and characterization of l-Alanine Potassium Nitrate Single Crystals for Nonlinear Optical Applications [14]; Synthesis and characterization of lanthanum chloride-doped l-Alanine maleate single crystals [15]; Growth, morphology, optical, thermal, mechanical, and electrical studies of a Cesium chloride-doped l-Alanine single crystal [16]; Bulk growth and characterization of semi-organic nonlinear optical l-Alanine cadmium chloride single crystal by modified Sankaranarayanan–Ramasamy method [17]; and Growth and Characterization of Pure and Thiourea-Doped l-Alanine Single Crystals for NLO Devices [18]. Among all the literature surveys and the author's best of knowledge, there was no record on Zirconium-doped l-Alanine single crystals. In the present investigation, authors have grown pure l-Alanine and Zirconium Nitrate-doped l-Alanine single crystals and presented their structural, spectral, optical, thermal, magnetic, dielectric, and photoconductivity analyses results in this article.
2 Experimental
2.1 Crystal growth
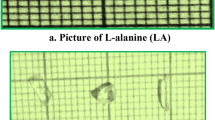
The solubility of l-Alanine (LA) was measured at different temperature and graph was plotted between temperature and Solubility which is shown in Fig. 1. The solubility data are very good agreement with literature values [19, 20]. The title compound was synthesized with 16.8 g of l-Alanine (99%, Merck) in 100 ml double-distilled water at room temperature as per solubility graph by low-temperature solution growth slow evaporation technique [21, 22]. It has stirred continuously for about 6 h to get saturated homogeneous solution. The obtained solution was filtered doubly with the help of No. 1 quality Whatman filter paper (11 µm porosity) for removing suspended impurity. The filtered LA solution was covered with perforated polythene sheet and kept unperturbed place to allow slow evaporation process. After 30 days, well defined and highly transparent good quality of l-Alanine single crystal was harvested with dimensions of 13 × 5 × 4mm3 (Fig. 2a).
As per above said procedure have followed to grow Zirconium Nitrate-doped l-Alanine (ZNLA) single crystal. l-Alanine (16.8 g) and 0.1 mol% of Zirconium Nitrate (3.4 g) were dissolved in 100 ml double-distilled water at room temperature. Then, the solution was continuously stirred for about 6 h with the aid of magnetic stirrer to get homogeneous and completely saturated solution. The obtained solution was doubly filtered by No. 1 quality Whatman filter paper and covered with perforated polythene sheet. Then, it was kept dust free and undisturbed place to slow evaporation. After a period of around 45 days, a clear transparent and colourless good quality ZNLA crystal was harvested with the dimensions of 20 × 5 × 4mm3 (Fig. 2b). Quality of both LA and ZNLA was improved by recrystallizing the grown crystals for three times. The as-grown crystals were further studied by various characterization techniques such as X-ray diffraction, UV–Visible, FTIR, Magnetic and dielectric studies, SHG, and Thermal and Photoconductivity analyses.
2.2 Characterization techniques
Lattice parameters and space group were calculated using powder (X’Pert PRO PANalytical) X-ray diffractometer with CuKα radiation (λ = 1.5406 Ả) and single-crystal (Enraf-Nonius CAD4 diffractometer) X-ray diffraction analysis with Mo-Kα radiation (0.71073 Å). Optical transmission and absorption spectra were carried out by using PerkinElmer EZ301 UV–Visible spectrophotometer. The reliable information on the chemical bonding and molecular structure of the compound was analyzed by FTIR spectra using PerkinElmer Spectrometer. The magnetic studies were performed by using Lakeshore VSM 7410 Vibration Sample Magnetometer (VSM). Thermal analysis was carried out by using PerkinElmer TGA 4000 analyzer under the nitrogen atmosphere and heating rate of 20̊ C/minute. Relative SHG efficiency was calculated by using the Kurtz powder technique with Nd:YAG laser wavelength 1064 nm. Photo and dark current was measured by KEITHLEY PICOMETER in the presence of a DC electric field at room temperature. The dielectric constant and dielectric loss were estimated by using HIOKI 3532-50 LCR HI Tester.
3 Result and discussion
3.1 Single-crystal X- ray diffraction
The Crystal system and space group of the LA and ZNLA crystals were identified and confirmed by single-crystal X-ray diffraction studies. The evaluated lattice parameters affirmed that both LA and ZNLA have a place to the framework of orthorhombic with the space group P212121. ZNLA lattice parameters have also marginally extended due to the amalgamation of Zirconium Nitrate in l-Alanine. The space group had also clearly showed that harvested crystal is a noncentrosymmetric pre-requisite for nonlinear optical applications [23]. The estimated lattice parameters are presented in Table 1. All the lattice parameters well comprehended with the already reported values [24,25,26].
3.2 Powder X- ray diffraction
The crystal structure and the Bragg’s peaks for the finely powdered LA and ZNLA samples were identified and exhibited in Fig. 3. All the peaks were ordered with the help of JCPDS card no. 08-0483. From Fig. 3 it is clear that the peak intensities and diffraction points were shifted slightly due to Zirconium doping (ionic span = 0.080 nm) in l-Alanine without any alteration in its crystal structure.
The obtained result of powder X-ray diffraction information of the prepared materials was in good agreement with the results with literature data [27]. From Fig. 3, the crystallite size was calculated by using Scherrer equation [28,29,30],
where D is the crystallite size, K is a constant (0.9), λ is X-ray wavelength, θ is the Braggs angle, and β is the full width at half maxima value. The calculated average crystallite size of the materials is 53.21 nm for LA and 62.17 nm for ZNLA. The lattice strain for LA is 0.0048 for LA and 0.0041 for ZNLA sample. The calculated ZNLA crystallite was higher than that of pure LA due to the incorporation Zirconium Nitrate dopant in l-Alanine. Dislocation density of the material was estimated by using the relation (2).
where, δ is the dislocation density and D is the crystallite size. The dislocations are influenced by physical properties such as optical quality, thermal stability, mechanical stability, and electrical properties. The nonlinear optical device depends on high-quality single crystals because the defects generated during growth which cause the distortion in the optical beam. Therefore, it is very important to grow the single crystals with reduced dislocation density for nonlinear optical device applications [31]. The calculated dislocation densities of l-Alanine and Zirconium-doped l-Alanine crystals are 3.532 × 10–4 nm−2 and 2.587 × 10–4 nm−2, respectively. The lower dislocation density of doped crystals was specified as having a much more crystalline nature even than pure LA [32]. Hence, Zirconium-doped l-Alanine crystal is more suitable for nonlinear optical applications.
3.3 FTIR spectral analysis
Fourier transform infrared spectroscopy gives more data of the materials like modes of vibration, functional groups, and structure [33, 34]. Fourier transform infrared spectra of LA and ZNLA materials are recorded and shown in Fig. 4. The presence of NH2 is due to the addition of hydrogen to the COOH group. The absorption peak at 3108 cm−1 indicates the presence of the NH3+ amine group and OH group in the grown crystal and there is a symmetrical extended CH3+ band at 3051 cm−1. The peak at 1535 cm−1 was attributed to C–N asymmetrical stretching of auxiliary amines. The solid band at 1169 cm−1 is due to the asymmetrically coupled vibration of alanine. The spectrum exhibits NH3 and CH3 rocking frequency range between 1137 and 1076 cm−1. The strong absorption peak at 502 cm−1 is contributed to symmetrical bending of S–C–N and NH3+ torsion. There are COO wagging and bending frequencies observed at 658 cm−1 and 552 cm−1 [35]. In this analysis, Zirconium Nitrate-doped l-Alanine material peaks were marginally moved due to Zirconium Nitrate in pure l-Alanine.
3.4 UV–visible studies
UV–Visible studies are considered as the most imperative one within the optical application point of view, it gives information about various optical parameters, such as absorption, transmittance, lower cutoff wavelength, and band gap [29]. The recorded transmittance and absorption spectra of LA and ZNLA crystal are depicted in Fig. 5a and b. Both LA and ZNLA crystals have high transmittance in the wavelength range between 250 and 800 nm for LA and 350–800 nm for ZNLA which were found to be 93% and 97%, respectively. The transmittance is essential and important for frequency conversion device applications. Therefore, owing to high transmittance, the doped LA crystal is a well appropriate material for NLO applications.
Optical absorption spectra provide information about the progression of electrons in σ and π orbital from the ground state to a higher energy state. The least absorption spectra of the grown materials (Fig. 5b) are due to the zwitterions of the carboxyl group that represents the n–π* transition (n—electrons advanced to antibonding π orbital). According to the reported values (LAT–235 nm: LAPN–272 nm) [12, 14], similarly here due to extra concentration of Zirconium Nitrate, the lower cutoff wavelength of ZNLA crystal (328 nm) was found to be higher than that of pure LA crystal (242 nm). The direct and indirect permitted transition band gap values for LA and ZNLA materials were analyzed using the Tauc plot and shown in Fig. 5c. The direct permitted band gap values are 5.4 eV and 4.84 eV for LA and ZNLA, and the indirect band gap values are 5.32 eV and 4.11 eV for LA and ZNLA crystals, respectively. The measured band gap values are highly agreement with literature data [14,15,16]. Consequently, the obtained results confirmed that the grown ZNLA crystal is a remarkably well reasonable one for Photonics and optoelectronic applications [35,36,37].
3.5 Second harmonic generation studies
A Q-switching high-energy Nd:YAG laser radiation with wavelength of 1064 nm, power input 27.43 mW, and 300 microseconds pulse duration with a repetition rate of 10 Hz was used to confirm nonlinear optical nature of the grown LA and ZNLA single crystals. Laser radiation exposed to the powdered samples of LA and ZNLA, bright green emission radiation (λ = 532 nm), was observed as output, which clearly expresses that grown both LA and ZNLA materials have nonlinear optical nature. The calculated relative second harmonic efficiency of LA and Zirconium Nitrate-doped l-Alanine compounds are 1.15 and 1.47 times higher than that of KDP. The increase in the second harmonic efficiency of the doped material is due to the presence of a transition metal dopant in the crystal lattice [38]. SHG efficiency of doped l-Alanine has a higher value than that of the pure l-Alanine owing to the presence of Zirconium Nitrate in l-Alanine. It is in good agreement with the already presented values (LA–0.85: LAT–1.18: LAPN–1.25) [12, 15, 16]. The results disclose that Zirconium Nitrate-doped l-Alanine single crystal is an appropriate candidate for NLO applications [39].
3.6 Dielectric studies
The dielectric analysis was performed to determine the different electrical parameters of the materials by changing the frequency with the temperature and the interconnection between the electro-optical properties of the crystals. The behavior of the atoms, ions, and their bonding can also be characterized by dielectric constant. The dielectric constant of LA and ZNLA crystals was accurately measured using the relation (3) with frequency variance at room temperature and shown in Fig. 6a.
where C—capacitance of parallel plate capacitor (Farad), d—the thickness of the material (mm), ɛ0—the permittivity of the free space (8.85 × 10–12 C2N−1 m−2), and A—area of the material (m3). The estimated dielectric constants of LA and ZNLA are 68 and 43 at low frequency, 4 and 4.5 at high frequency. The high values of εr at low frequencies may be due to the excitation of bound electron and the presence of all the four polarizations namely, space charge, oriental, ionic, and electric polarization and its low value at high frequencies may be due to the loss of significance of this polarization gradually. The motion of the dipole could not alter its direction after certain frequencies with an applied electrical field, which is the representation of a decrease in polarization. Materials with a high dielectric constant have large dipoles and, as a result, might have a maximum loss. Figure 6b indicates the frequency variation of dielectric loss in the LA and ZNLA samples where the dielectric loss decreases and meets nearly constant with increasing frequency. It clearly shows that such materials have an optical quality enhancement that is desirable for NLO applications.
The low dielectric loss and dielectric constant at high frequency, therefore, suggest that crystal obtained will be of good optical quality with a fairly small defect that is of high value for nonlinear optical materials applications such as optoelectronics industries and electro-optical modulators [40, 41]. As per the Miller rule, a low value of the dielectric constant at a higher frequency is an ideal parameter for the enhancement of SHG efficiency.
3.7 Thermal Studies
Thermal properties are an essential one for the nonlinear optical device fabrication. Thermal stability, weight losses, and endothermic nature of the grown material were studied by thermal analysis [42, 43]. TGA/DTA curves of LA and ZNLA crystals are shown in figs. 7 and 8. l-Alanine and Zirconium Nitrate-doped l-Alanine single crystal are thermally stable up to 193.5 35 °C and 248.35 °C. The observed maximum weight losses are 88% between 193.5 and 284.60 °C for LA and 82% between 248.35 and 318.25 °C for ZNLA due to liberation of gases like CO2 and NH3 [44,45,46,47]. From the DTA curve, the endothermic peak of the LA and ZNLA crystals was seen at 281.68 °C and 285.54 °C, which specifies the melting point of the materials. The endothermic peak observed in DTA indicates that the grown materials have a high degree of crystallinity [48]. Thermal stability and melting temperature of Zirconium Nitrate-doped l-Alanine crystal have a higher value than that of pure l-Alanine which is confirmed from the already-reported values ( LaCl3-doped LAM and LACC: thermal stability—145 °C & 248 °C and Melting temperature—149 °C & 285 °C [15, 16]. The results disclose that Zirconium Nitrate-doped l-Alanine single crystal is an appropriate candidate and could be used up to 248.35 °C for NLO applications.
3.8 Photoconductivity analysis
Photoconductivity study was mainly used to measure the electrical conduction of the grown material with and without the supply of photonic radiation by varying DC voltage between 2 and 8 V [49]. Initially, the dark current for LA and ZNLA crystals was noted by varying DC input voltage from 2 to 8 V in the absence of light radiation. Then, photocurrent was measured by applying light radiation (100 W Halogen lamps) on LA and ZNLA by varying the DC input voltage between 2 and 8 V. The graphs were plotted between DC input voltage and current (dark current and photocurrent) and are shown in Fig. 9. The value of dark current for both the LA and ZNLA crystals was higher than photocurrent which reveals that both the materials have the nature of negative photoconductivity. Negative photoconductivity behavior occurs in solid crystalline materials and owes to minimize the mobile carriers in the presence of light radiation [11, 32]. The suppression of mobile carriers of the conduction band and the annihilation of minority carriers in the valence band leads to the result of negative photoconductivity [50,51,52,53]. The photosensitivity of the grown materials was calculated by using Eq. (4),
where Id is the dark current and Ip is the photocurrent. The calculated photosensitivity of the LA and ZNLA are 0.5385 and 0.4378 at 2 V. Due to the lower value of photosensitivity, the Zirconium Nitrate-doped material is suitable for nonlinear optical applications.
3.9 Magnetic properties
The magnetic properties of the grown crystals were inspected by Vibrating Sample Magnetometer (VSM) at room temperature. Figure 10 shows the hysteresis loop of magnetization of the material against the correlated magnetic field. The method of hysteresis loop magnetization of the material indicates that it is additionally ferromagnetic for the associated field. The process of ferromagnetism tends to occur in the materials of atoms or particles that had a permanent magnetic moment as a result of filled shells or unpaired electrons [54, 55]. The estimated values of magnetization saturation, coercivity, and retentivity for l-Alanine and Zirconium Nitrate-doped l-Alanine are presented in Table 2. The estimated retentivity and magnetization are 25.444 × 10–6 emu, 127.10 × 10–6 emu for LA, and 68.559 × 10–6 emu, 225 × 10–6 emu for ZNLA single crystals.
By including the dopants in small amounts, so that the atoms arbitrarily involve substitution positions, we obtain a small drop in the value of the saturation magnetization for pure LA material comparing ZNLA material and this is due to a local displacement of the spin–orbit coupling, resulting in reduced magnetic anisotropy energy. These are inherent impacts, which reflect in an altar of the coercivity. It occurs since the coercive field essentially comes from the irregular changes in the energy of the traveling domain walls, interacting with the defective structure of the material. The coercivity of LA and ZNLA is 6542.9 C and 3516.9 C, respectively. Coercivity is the area that requires magnetization to be limited to the extent of zero. The pure LA material is called hard ferromagnetic because of its strong coercivity ferromagnetic nature. The ZNLA crystal is called soft ferromagnetic because of its weak coercivity ferromagnetic nature. The magnetization value of doped material has higher than that of pure LA due to Zirconium Nitrate in LA. The obtained results clearly express that magnetic properties of the grown material was dependent varied due to variation of crystallite size. The high ferromagnetic content will have a small micro-crystalline anisotropy and fewer defects than the crystal grain. As a result, it is clear that Zirconium-doped l-Alanine crystal can be a gentle ferromagnetic material and it can be well suited for optical storage applications.
4 Conclusion
High-quality single crystal of l-Alanine (13 × 5 × 4 mm3) and Zirconium Nitrate-doped l-Alanine (20 × 5 × 4 mm3) were grown at room temperature using the slow evaporation method. The obtained lattice parameters from single-crystal XRD studies confirmed that the LA and ZNLA crystals belong to the orthorhombic system with space group P21 2121. Functional groups of the samples have confirmed by FTIR analysis. 7 UV–Visible- NIR spectroscopy confirms that the Zirconium Nitrate-doped l-Alanine crystal (97%) has higher optical transparency than pure l-Alanine crystal (93%) and the lower cutoff wavelength at around 242.8 nm for LA, 328.57 nm for ZNLA crystals. Its direct optical band gap value calculated to be 5.4 eV for LA and 4.84 eV for ZNLA materials. The second harmonic efficiency of l-Alanine and Zirconium Nitrate-doped l-Alanine compounds is 1.15 and 1.47 times higher than that of KDP. The value of dielectric constant was low at high frequency and the low value of dielectric loss reveals that the grown crystals are of good quality with fewer defects and hence with crystalline perfection. The estimated retentivity and magnetization are 25.444 × 10–6 emu, 127.10 × 10–6 emu for LA, and 68.559 × 10–6 emu, 225 × 10–6 emu for ZNLA single crystals. Both LA and ZNLA crystals have the nature of photo negativity and the calculated photosensitivity of LA and ZNLA are to be 0.5385 and 0.4378 at 2 V respectively. The observed melting temperature of LA and ZNLA is 281.68 °C and 285.54 °C. Hence, all these analyses indicate that the grown ZNLA crystal is the potential material for SHG as well as a promising candidate to be the suitable one for photonic and nonlinear optical device applications.
References
M. Iwai, T. Kobayashi, H. Furuya, Y. Mori, T. Sasaki, J. Appl. Phys. 36, 276 (1997)
D.R. Yuan, D. Xu, N. Zang, M.G. Liu, M.H. Jiang, Chain Phys. Lett. 13, 841 (1996)
S. Sathiyanathan, M. Selvapandiyan, Mater. Sci. Pol. 37, 615 (2019)
S. Chandran, R. Paulraj, P. Ramasamy, Opt. Mater. 52, 49 (2016)
Y. Goto, A. Hayashi, Y. Kimura, M. Nakayama, J. Cryst. Growth. 108, 688 (1991)
S. Velayutham, M. Selvapandiyan, Heliyon 5, e02091 (2019)
R.W. Boyd, Nonlinear Optics (Academic Press, San Diego, Calif, USA, 1992)
K. Omri, A. Bettaibi, K. Khirouni, L. El Mir, Phys. B 167, 53715 (2018)
K. Omri, F. Alharbi, Appl. Phys. A 125, 696 (2019)
K. Omri, I. Najeh, L. El Mir, Ceram. Int. 42, 8940 (2016)
N. Suresh, M. Selvapandiyan, Bull. Mater. Sci. 43, 166 (2020)
K. Rajesh, B. Milton Boaz, P. PraveenKumar, J. Mater. 2013, 613092 (2013)
G. Marudhu, S. Krishnan, T. Thilak, P. Samuel, G. Vinitha, G. Pasupathi, J. Nonlinear Opt. Phys. Mater. 22, 1350043 (2013)
M. Shanmuga Sundaram, V. Vijayalakshmi, P. Dhanasekaran, O.N. Balasundaram, S. Palaniswamy, J. Cryst. Growth 506, 122 (2019)
D. Alemu Fentawa, M. Esthaku Peterb, T. Abzaa, J. Cryst. Growth 522, 1 (2019)
P.M. Wankhade, G.G. Muley, Chin. J. Phys. 55, 2181 (2017)
C. Justin Raj, S. Jerome Das, Cryst. Growth & Des. 8, 2729 (2008)
P. Malliga, A.A.J. Pragasam, J. Rus. Las. Res. 34, 346 (2013)
M. Shkir, I.S. Yahia, A.M.A. Al-Qahtani, V. Ganesh, S. Alfaify, J. Mol. Strut. 1131, 43 (2017)
T. Raghavalu, G. Rameshkumar, S. Gokul Raj, V. Mathivanan, R. Mohan, J. Cryst. Growth 307, 112 (2007)
P. Ramasamy, P. Santhana Raghavan, Crystal Growth Processes and Methods (Kru Publications, Kumbakonam, India, 1999)
J.C. Brice, Crystal Growth Process (Wiley, New York, 1986)
M. Ahlam, B. Hemaraju, A.G. Prakash, Optik 124(23), 5898 (2013)
U. Charoen-In, P. Ramasamy, P. Manyum, J. Cryst. Growth 312, 2369 (2010)
L. Misoguti, A.T. Varela, F.D. Nunes, V.S. Bagnato, F.E.A. Melo, F.J. Mendes, S.C. Zilio, Opt. Mat. 6, 147 (1996)
S. GokulRaj, G. Ramesh Kumar, Adv. Mater. Lett. 2, 176 (2011)
N. Vijayan, S. Rajasekaran, G. Bhagavannarayana, R. Ramesh Babu, R. Gopalakrishnan, M. Palanisamy, P. Ramasamy, Cryst. Growth Des. 6, 2441 (2006)
M. Lydia Caroline, R. Sankar, R.M. Indirani, S. Vasudevan, Mater. Chem. Phys. 114, 490 (2009)
K. Rajesh, A. Mani, K. Anandan, P. PraveenKumar, J. Mater. Sci. Mater. Electron. 28, 11446 (2007)
G.K. Williamson, R.E. Smallman, Philos. Mag. 1, 34 (1956)
V. Sivasubrramani, V. Mohankumar, M. Senthilpandian, P. Ramasamy, CrystalEngComm (2017). https://doi.org/10.1039/C7CE01202K
J. Arumugam, M. Selvapandiyan, C. Senthilkumar, M. Srinivasan, P. Ramasamy, J. Mater. Sci. 31, 6084 (2020)
G. Bhagavannarayana, S. Parthiban, S. Meenakshisundaram, Cryst. Growth. Des. 8, 446 (2008)
T. Uma Devi, N. Lawrence, R. Ramesh Babu, K. Ramamuthi, J. Miner. Mater. Charact. Eng. 8, 755 (2009)
D. Jaikumar, S. Kalainathan, G. Bhagavanarayana, J. Cryst. Growth 312, 120 (2009)
G.K. Priya Merline, M. Chitra, J. Mater. Sci. 29, 5509 (2018)
M. Anbuchezhiyan, A. Arputhalatha, S. Ponnusamy, K.S. Suresh Babu, Photon. Lett. Pol. 7, 44 (2015)
P. Praveen Kumar, V. Manivannan, S. Tamilselvan, S. Senthil, V.A. Raj, P. Sagayaraj, J. Madhavan, Opt. Commun. 281, 2989 (2008)
B.S. Benila, K.C. Bright, S. Maey Delphine, R. Shabu, J. Magn. Magn. Mater. 426, 390 (2017)
N. Kanagathara, G. Anbalagan, Int. J. Opt. 2012, 1 (2012)
S. Sagadevan, P. Murugasen, Int. J. Mater. Sci. Eng. 3, 159 (2015)
K. Nivetha, W. Madhuri, Opt. Laser. Technol. 109, 496 (2019)
S. Mukherjee, V. Sudarsan, R.K. Vasta, A.K. Tyagi, J. Lumin. 129, 69 (2009)
M.K. Sangeetha, M. Mariappan, G. Madhurambal, S.C. Mojumdar, J. Therm. Anal. Calorim. 112, 1059 (2013)
M. Prakash, D. Geetha, M.L. Caroline, P.S. Ramesh, Spectrosc. Chem. Part A 83, 461 (2011)
S. Natarajan, S.A. Martin Britto, E. Ramachandran, Cryst. Growth Des. 6, 137 (2006)
D.P. Ketan, J.D. Dipak, J.J. Mihir, Mod. Phys. Lett. B 23, 1589 (2009)
T. Thilak, M. Basheer Ahamed, G. Marudhu, G. Vinitha, Arab. J. Chem. 9, 676 (2016)
T. Ananthi, S.M. Delphine, A.W. Almusallam, Recent Res. Sci. Technol. 3, 32 (2011)
P. Baskaran, M. Vimalan, P. Anandan, G. Bakiyaraj, K. Kirubavathi, K. Selvaraju, J. Taibah Univ. Sci. 11, 11 (2017)
J. Jeyaram, K. Varadharajan, B. Singaram, R. Rajendhran, J. Sci. 2, 445 (2017)
P. Baskaran, M. Vimalan, P. Anandan, G. Bakiyaraj, K. Kirubavathi, K. Selvaraju, J. Taibah Univ. Sci. 11, 11 (2018)
K. Pandurangan, S. Suresh, J. Mater. 2014, 1 (2014)
C. Karnan, A.R. Prabakaran, M. Prabhaharan, G. Vinitha, J. Electron Mater. 48, 7915 (2019)
F. Akhtar, J. Podder, Res. J. Phys. 6, 31 (2012)
Acknowledgements
Authors thank Head, Department of Physics, Alagappa University, Karaikudi for extending the characterization facilities of Powder XRD and also thank The Head, SAIF, IIT, Chennai for UV–visible analysis and VSM analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suresh, N., Selvapandiyan, M. Influence of Zirconium Nitrate doping on the properties of l-Alanine crystal for nonlinear optical applications. J Mater Sci: Mater Electron 31, 16737–16745 (2020). https://doi.org/10.1007/s10854-020-04229-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04229-2