Abstract
A systematic investigation of the effect of Strontium (Sr) doping on the structure, magnetic behavior, and magnetocaloric properties of the manganite perovskites \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) (LCSMNO) has been performed. The Sr content x takes three values, namely 0.15, 0.2, and 0.3. The sol–gel technique has been employed in the preparation of our compounds. The structural analysis has revealed that our samples exhibit a rhombohedral structure with \(R\bar{3}c\) space group symmetry, close to ambient temperature. A second-order paramagnetic (PM) to ferromagnetic (FM) transition has been validated over the Curie temperature range TC between 319 K (x = 0.15) to 353 K (x = 0.3), based on Arrott's analysis with the behavior of the master curve. According to the calculations made, the maximal values of the magnetic entropy change \({(-\Delta S}_{M}^{max})\) resulting through Landau's theory and the ones obtained by the classic Maxwell relationship are very close. We find the value of \({(-\Delta S}_{M}^{max})\) is equal to 4.386, 4.469, and 4.250 J Kg−1 K−1, respectively, for x = 0.15, 0.2, and 0.3, when an external magnetic field μ0H = 5 T is applied. Based on the results of the study in this work, we find that controlled Sr doping adapts perfectly to the magnetic as well as the magnetocaloric properties of the LCMNO, which plays an important role in the implementation of many potential applications of the material in the field of near-ambient temperature magnetic refrigeration technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Several scientific research teams have extensively investigated perovskite manganese oxides. The general formula of this mineral is Ln1−xAxMnO3. It contains a rare-earth Ln and a monovalent alkali metal or a divalent alkaline earth A [1,2,3,4,5]. These materials have a variety of phases as well as a very important physical property. Since 1993, subsequently to the finding, in thin layers of La1−xCaxMnO3, of the colossal magnetoresistance (CMR) [6, 7], these manganites have been collected. This finding has a very attractive impact on fundamental studies as well as on industrial applications, such as the manufacturing of high storage capacity hard disks and reading heads. The new magnetic refrigeration application has received much attention in recent years. If an external magnetic field is applied or absent, the magnetic solids are heated or cooled. Thanks to its several advantages over conventional gas refrigeration, namely low vibrations and noise, no harmful gas emission and competitive technology, this application is described as an emerging technology. It takes advantage of the magnetocaloric effect.
The Gd presents the best magnetic entropy change − ΔSM value that equals 10.2 J/K kg [8] and a Curie temperature TC of 294 K under an applied magnetic field of 5 T. Unfortunately, Gd is very expensive and oxidizable in air. For this reason, research is oriented toward the synthesis of new materials with these magnetocaloric properties near room temperature at reasonable costs.
The current study of perovskite manganites has been stimulated by the possible use of their significant magnetocaloric (MC) properties at ambient temperature and at weak magnetic fields [9, 10]. The MC effect has been studied on a series of massive perovskite manganites LaAgMnO3 by Tang et al. who discovered a better change in magnetic entropy than that of gadolinium [11, 12]. Therefore, the use of these materials as magnetic refrigerants that work over a very large temperature range is possible. Based on this point of view, this study focuses on the effects of Sr substitution on the magnetic in addition to the magnetocaloric properties in LCSMNO with x = 0.15, 0.2, and 0.3. The compounds were produced using the sol–gel method. Subsequently, we produce a material with very advantageous parameters, for example, no corrosion, easy synthesis, and chemical stability as well as low cost, exhibiting a colossal magnetocaloric effect close to room temperature.
2 Experimental details
The sol–gel method have been used to prepare the \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) compounds. This technique was described in our previous studies [13]. Three values of the content x are used, namely 0.15, 0.2, and 0.3. In addition, the production of good quality samples has been made by the use of sol–gel method thanks to its various advantages such as easy preparation, excellent homogeneities, low temperature treatment, simplicity of manufacture, a detailed microstructure and chemical control, and fast annealing times [14]. To form various classes of materials such as ceramics, films or thin fibers as well as glasses, this technique has been widely used.
3 Results and discussion
3.1 Structural properties
X-ray diffraction is a widely used method for determining the nature, crystal structure, and lattice parameters of samples. It also allows the identification of the various phases formed and their range of existence as well as to assess the purity of the sample.
A monochromatic and parallel incident X-ray beam penetrates a crystal containing an ordered periodic arrangement of atoms in equidistant, more or less dense three-dimensional reticular planes, which are indicated by their coordinates (h, k, and l). The X-ray crystal interaction causes the diffracted beams to appear in a specific direction. This result is verified only if the diffraction condition is satisfied, that is the radiation wavelength must be the same order of magnitude as the interatomic distances and that this radiation encounters planes at a certain angle θ called the Bragg angle. According to the Bragg’s law [15]:
where \({d}_{\text{hkl}}\) is the spacing between the planes in the atomic lattice, \({\text{sin}}\,\theta\) is the angle between the incident ray and the scattering planes, n is an integer and \(\lambda\) is the wavelength of the incident wave; therefore, the decreasing \({d}_{hkl}\) lead to the increasing h value [16].
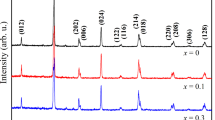
From X-ray diffraction patterns obtained at room temperature, it can be seen that all components crystallize in the rhombohedral structure with the space group R \({\bar{3}}\) c.A BS2 magnetometer has been used to perform the magnetic measurements. More precisely, the magnetization has been measured as a function of the applied magnetic field in the vicinity of Curie temperature TC.
In Fig. 1, we have represented the X-ray powder diffraction patterns of the polycrystalline compounds \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) (x = 0.15, 0.2, and 0.3). There is no detectable secondary phase in any of the compounds. From diffraction peak indexing, the perovskite structure is rhombohedral with \(R\bar{3}c\) space group symmetry. In Table 1, we have grouped the structural parameters. Since the average radius of Sr2+ is larger than that related to Ca2+ [17], the unit cell volume per formula ranges from 345.922 to 348.768 Å when x increases from 0.15 to 0.3. This results from the optimization of the average A-site radius \(\langle {r}_{A}\rangle\) , respectively, from 1.368 to 1.383 Å which leads to the production of MnO6 octahedron distortion. It should be mentioned that the Mn–O–Mn angle equals 180° in the perfect perovskite structure. In the present work, there is only the doubling of peaks (100) and (104) of rhombohedric symmetry \(R\bar{3}c\) for all compounds, as Sr content increases. In the matter of the results available in the literature related to samples with x ranging from 0.055 to 0.110 [18], they have revealed mixed orthorhombic and rhombohedral phases.
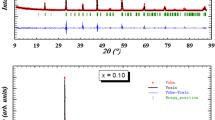
Figure 2 illustrates the Rietveld analysis results related to the three investigated samples. Observed, calculated and differential XRD diagrams are represented. Observed and calculated intensity differences are depicted by the solid green line in the diagram, as well as the positions of the Bragg reflection are expressed by vertical bars. Based on reliability weighting factors Rwp, which are equal to 10.2%, 9.56%, and 9.15%, respectively, for x = 0.15, 0.2, and 0.3 compounds, the results of the adjustment are in good agreement. In Table 1, we have listed in detail the different Rietveld refinement results for all compounds.
Figure 3a summarizes the cell parameters versus Sr substitution. As Sr dopant content increases, we find that the lattice constants a = b and c increase monotonically with the cell volume V. From the inset of Fig. 3, the increase in cell volume V as a function of Sr doping is expected because the ionic radius Sr2+ is greater than that related to Ca2+.
Moreover, the average grain size Dsc can be estimated based on XRD patterns according to Scherrer formula [19]:
where λ is the X-ray wavelength being used (λ = 0.15406 nm), θ denotes the strongest peak diffraction angle and β shall be defined as follows: β2 = β2m–β2s. In our case, βm can be defined with the experimental full width at half maximum (FWHM) and βs is the FWHM of a standard silicon sample.
Figure 4 illustrates the surface morphology as well as the grain size of \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) (x = 0.15, 0.2, and 0.3) samples using SEM microscope. Knowing that, the presence of a polycrystalline nature of the compound is revealed by micrography [20]. For this reason, we have shown in Fig. 4, the size distribution of compounds with image J software. As it has already been shown, there is a good modeling of the histogram by Gaussian function. The average crystallite size of the samples was indicated by the Gaussian fitting (see Table 1). From the obtained results, we have observed that the average grain size values obtained through SEM micrographs are significantly higher than the ones estimated by XRD data. This difference can be explained by the constitution of each observed particle in SEM by multiple crystallite domains, caused by defects (vacations, dislocations) or by internal stresses inside the particle [20].
3.2 Magnetic characterization
The variation of magnetization M versus temperature under an applied magnetic field of 0.05 T for \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) compounds are shown in Fig. 5. Clearly, we agree that all samples indicate the net paramagnetic to ferromagnetic (PM–FM) transition.
Indeed, Curie temperature can be expressed based on the log-normal weighted Langevin function using the following formula [21, 22]:
where \({M}_{0}\) is the magnetization at (T = TC), C is a proportionality term, \((coth\left(T-{T}_{C}\right)-\frac{1}{\left(T-{T}_{C}\right)})\) is a secondary term canceling out at (T = TC). We have fitted curves to get the TC values, which are equal to 319, 330, and 350 K for \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) for x = 0.15, 0.2, and 0.3, respectively (see Fig. 5). In Table 2, we have recorded the Curie temperatures of all samples, which are given using the minimum of the derivative (dM/dT). For the \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\)(x = 0.15, 0.2, and 0.3) polycrystalline, the TC values are equal to 318, 331, and 352 K, in respective way, which is in good agreement with the values given in refs.[23, 24]. In addition, those results are consistent with those obtained from the Langevin function fitting. During the increase of Sr doping from 0.15 to 0.30, this explains that TC increases briefly from 318 to 352 K. Possible structural factors influencing the increase of TC are Mn3+ /Mn4+ ratio, the tolerance factor (τ), the bond length (dMn–O), the bond angle Mn–O–Mn (ӨMn–O–Mn), the size mismatch factor of A-site cations (σ2) that comes out from the doping of Sr2+ and Ca2+ in the La3+ site, and the average ionic radius in A-site \(\langle {r}_{A}\rangle\).In the case of \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\), the parameters \(\langle {r}_{A}\rangle\) and σ2are determined by the following Eqs. (4) and (5):
Knowing that, the factor \({\sigma }^{2}\) describes the distribution of cations over the A sites as well as their random disorder, on which \({y}_{i}\) expresses the fractional occupancy of the A-site ion and \({r}_{i}\) corresponds to the ionic radius. From Shannon [25], we have derived ionic radii of related cations using twelve coordinate radii for the A-site ions. The obtained values corroborate with those related to a deformed lattice in the manganite perovskites. In Table 2, we have listed all the parameters \(\langle {r}_{A}\rangle\) and \({\sigma }^{2}\) of all the samples.
This can be seen quantitatively and more in Fig. 6a–c, where the derivatives of magnetization as a function of temperature (dM/dT) are presented for all compounds. Using a Gaussian function with a Lorentzian function, we have fitted peaks to get the TC values. Considering that Lorentzian adjustments are always preferable to data and that TC, determined from the adjustment are shown in Table 2. In Fig. 6d, we have represented the plot of TC versus \(\langle {r}_{A}\rangle\). Thereafter, we see that TC grows monotonically with increasing \(\langle {r}_{A}\rangle\).The most interesting result is shown in Fig. 6e, where TC rises linearly with the A-size mismatch factor \(\langle \sigma \rangle\). It appears clearly that \(\langle {\sigma }^{2}\rangle\) stands out as one of the main factors governing the magnetic transition temperature (TC) of manganite perovskites.
Derivatives of the magnetization (dM/dT) as well as the fitted Lorentzian function curve (line) of compounds with x = 0.15 (a), x = 0.2 (b) and x = 0.3 (c). d Average cationic radius dependency \(<{r}_{A}>\) on temperature TC. The line is a curve adjusted by a linear equation with R = 0.9939. e Variation of TC temperature as a function of A-site mismatch \(<{\sigma }^{2}>\). The obtained curve is fitted according to the linear equation with R = 0.9872
The growth of TC is due to the increase of x which leads to an increase of the average A-site ionic radius rA (see Fig. 6d), resulting in a stronger magnetic exchange interaction between Mn3+ and Mn4+, while favoring the FM order resulting in a shift of Curie temperature toward higher temperatures [26, 27]. We can also relate the variation of TC to the A-site disorder \({\sigma }^{2}\). Evidently, it can be observed that TC increases when \({\sigma }^{2}\) increases (see Fig. 6e). Such results were presented in Refs. [27, 28], in which authors demonstrate a linear increment of TC with \({\sigma }^{2}\) from studies of perovskites having a fixed rA.
Figure 7 exhibits the inverse of the magnetic susceptibility \(1/\chi\) versus the temperature when a magnetic field of 0.05 T is applied for \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) samples (x = 0.15, 0.2, and 0.3). The inverse of magnetic susceptibility (\({\chi }^{-1}\)) has a linear trend versus the temperature according to the Curie–Weiss law [29]:
Inverse susceptibility derived from magnetization measurements at 0.05 T field of the samples. The inset shows Sr content dependence of \({\theta }_{CW}\) and TC values for \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\)(x = 0.15, 0.2, and 0.3) compounds
where C is the Curie constant and \({}_{cw}\) is the Curie–Weiss temperature. These parameters result from adjusting the linear PM range of the data. The Curie constant is given by
where N is the Avogadro number, \({\mu }_{B}\) is the Bohr magneton and \({k}_{B}\) is the Boltzmann constant. Subsequently, the experimental effective moment (\({\mu }_{eff}^{exp}\)) was obtained. In Table 2, we have grouped all the values obtained of \({}_{cw}\) and \({\mu }_{eff}^{exp}\) of all compounds. An FM interaction between spins has been confirmed by the presence of positive \({}_{cw}\) values. The theoretical effective moment (\({\mu }_{eff}^{the}\)) value can be compared to the experimental value of (\({\mu }_{eff}^{exp}\)) using the formula below:
where \({\mu }_{eff}^{the}=5.59 {\mu }_{B}\) for \({Ni}^{2+}\), \({\mu }_{eff}^{the}=4.9 {\mu }_{B}\) for \({Mn}^{3+}and { \mu }_{eff}^{the}=3.87 {\mu }_{B}\) for \({Mn}^{4+}\)[9]
In the paramagnetic regime, we have noticed clearly that the measured effective moments (\({\mu }_{eff}^{exp}\)) are larger than the theoretical moments (\({\mu }_{eff}^{the}\)) (see Table 2). In general, this result is related to the existence of short-range FM correlation in the PM state [29].
In addition, we have presented in the inset of Fig. 7, the comparative analysis of TC and θcw versus Sr doping concentration. We observe that TC is almost linearly dependent on Sr content.
We have noticed that the difference between TC and θcw increases progressively with Sr content. This is explained by the progressive destruction of the ferromagnetic long-range order.
For magnetic field strengths up to 5 T, various magnetization curves dependent on the magnetic field (M–H) have been recorded at different temperatures around TC. Figure 8 depicts the isothermal magnetization M versus \({\mu }_{0}H\) for different temperatures and three compositions (x = 0.15, 0.2, and 0.3). As increasing the applied field, the isothermal magnetization curves of all components showed a saturation tendency with a non-linear behavior resulting in an extremely high increase of the measured magnetization at weak field values. However, we observed a tolerable decrease in magnetization and the non-linear isothermal magnetization curves turned almost linear at temperatures above TC. The reason for this reduction in magnetization is linked to thermal agitation, which causes damage to the magnetic moment arrangement. Globally, we have noticed that magnetization increases simultaneously with the increasing applied magnetic field, this is due to the rearrangement of the magnetic domains as well as a rise in the magnetic ordering.
Identification of the magnetic phase transition is carried out using the Banerjee criterion [30]. Following this criterion, the slope sign of H/M vs M2 curves (Arrott plots) is positive or negative which means a second-order or first-order magnetic phase transition, respectively. In the inset of Fig. 8b, d and f, we have shown the standard Arrott plots for \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) (x = 0.15, 0.2, and 0.3) samples. We then observed a positive slope close to TC in all specimens, which indicates the second-order magnetic phase transition.
Magneto-crystalline anisotropy has been described as an intrinsic property of materials playing a very significant part in the coercivity [31]. According to Stoner–Wohlfarth theory, the calculation of the anisotropy constant (Ka) is performed using [32]
The anisotropy constant decreases from 0.729 J/m3 for x = 0.2 to 0.422 J/m3 for x = 0.3. Knowing that, the saturation magnetization depends on an inverse way to the coercivity and the same behavior has been noted (see Fig. 9). The remanence ratio calculation was performed using [33]
From the obtained results, we notice that the remanence ratio increases from 0.032 for x = 0.2 to 0.049 for x = 0.3 (see Fig. 9). The small value of the remanence ratio indicates the isotropic nature of the compounds [33].
3.3 Magnetocaloric study
For measuring the efficiency of a magnetic refrigerator, the isothermal magnetic entropy change ΔSMis considered a key parameter [34]. Starting from the M–H curves illustrated in Fig. 8, we have calculated the ΔSM values from the following Maxwell's relationship:
The above integral can be computed using a numerical scheme since the magnetization has been measured over small variations of the adjustable parameters. It is straightforward to obtain the approximate relation:
where Mi represents the magnetization value at temperature Ti.
Figure 10 shows the variations of magnetic entropy exchange (− ΔSM) with the applied magnetic field (\({\mu }_{0}H\)) from 0 to 5 T at different temperatures are presented as a three-dimensional (3D) image for \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) (x = 0.15, 0.2, and 0.3) compounds. It is well known that the values of -ΔSM increase with the applied field as well as the maximum values (\({-\Delta S}_{M}^{max}\)) reach up to 4.38, 4.46, and 4.25 J/kg K for x = 0.15, 0.2, and 0.3, respectively, around the Curie temperature (see Table 3).
It may be noted that (\({-\Delta S}_{M}^{max}\)) is not the only parameter to determine the applicability of a material. To evaluate the suitability of a material for magnetic refrigeration (MR), Gschneidner and Pecharsky [38] have developed the Relative Cooling Performance (RCP), which is the main index used to assess the cooling efficiency of a magnetic refrigerant. This parameter refers to the product obtained from the maximum values of the magnetic entropy change (\({-\Delta S}_{M}^{max}\)) and the full width at half the maximum \(\delta {T}_{FWHM}\) of the magnetic entropy change curve (\(RCP={-\Delta S}_{M}^{max}\times \delta {T}_{FWHM}\)) [39]. In this context, this parameter represents the amount of heat exchanged between the cold and hot parts of the refrigerator during an ideal thermodynamic cycle. Table 3 summarizes the results.
The theoretical modeling of the magnetocaloric effect has been studied on the basis of Landau's theory, carried out by Amaral et al. [40, 41]. According to this theory, the free energy (G), related to the order parameter M, versus temperature, can be expressed as follows:
where A, B and C refer to the Landau parameters. They depend on the temperature. Similarly, that is also shown by the equilibrium condition of the magnetic system that is near the transition state (\(\frac{\partial G}{\partial M}=0\)). Here, close to the Curie TC temperature, the following equation is obtained:
The Landau coefficients have been determined according to Eq. 14 based on the experimental curve of the magnetic field variation versus magnetization M for a specific temperature.
After that, we obtained the magnetic entropy change from Eq. 12, which is given by [42]:
where \({A}^{^{\prime}}\left(T\right)\),\({B}^{^{\prime}}\left(T\right)\) and \(C^{\prime}\,({\text{T}})\) are the derivatives of the Landau coefficients.
As shown in Fig. 11, we present both the theoretical and experimental magnetic entropy change curves, which are derived from Landau's theory for x = 0.15, 0.20, and 0.3 compounds at an applied magnetic field of 5 T. We have displayed in the same figure the Landau parameters versus the temperature. For the A (T) curves, a minimum close to the transition temperature TC is found. Additionally, there was a second-order transition that has been confirmed by B (T) curves, which were shown as positive over TC and negative under it. Concerning C (T) values, they are very low in the order of 10–8. Furthermore, we note that both the experimental and theoretical curves indicate appropriate agreement over the TC with a small deviation below.
Some ferromagnetic domains found during the paramagnetic phase are responsible for this. It also ignores the potential effects of exchange interactions as well as the Jahn–Teller distortion. As a basis for determining the transition temperature, we consider the Landau parameter C (T) to be very low. In this case, for Eq. 14, we can ignore term 5, and we have the following equation:
The determination of A (T) has been performed in the linear region using a linear fit of Arrott plots (H/M vs. M2); it is an intercept parameter (insets in Fig. 12). Similarly, A (T) is expressed as A (T) = a1 (T − TC) around the Curie temperature [43]. In Fig. 12, we have illustrated the variation of A (T) versus temperature. As shown in the graph, A is negative for T smaller than TC whereas for T larger than TC it is positive. Assuming the intersection of A (T) curve and the equation a = 0, we have determined the Curie temperature that is 328 K, 339 K, and 361 K for x = 0.15, 0.2, and 03, correspondingly. It is very similar to the values found in experience.
Thermal magnetization, M (T) under various changes in the applied magnetic field has been shown in Fig. 13 for \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) (x = 0.15, 0.2, and 0.3) samples, respectively. These curves have been constructed with points that correspond to magnetic fields between 1 and 5 T in the isothermal analyses (Fig. 8).
When starting from the inset of Fig. 13, it can be seen that as the magnetic field increases, the transition gets wider and the dM/dT versus T-curve minima moves to a higher temperature. Depending on the result published in [44], with the increase of the applied magnetic field, the peak value of TC curve grows always bigger, for all values of ΔH, the maximum of -ΔSM is always located around the Curie temperature. In fact, explaining this behavior as the applied magnetic field increases, so the maximum of dM/dT moves considerably to higher temperatures, its magnitude disappears fast, resulting in the dominance of the weak field − ΔSM to TC.
The nature of the magnetic transition has been investigated using a phenomenological method, referred as the universal master curve in second-order phase transition components, as proposed by Franco et al. [45]. The basis of this method is the plotting of \({\Delta S}_{M}(T)\) curves, which are measured to various maximum applied fields, collapsing on a single master curve. The realization of the universal curve has been carried out using the normalized entropy change \({(\Delta S}_{M}/{\Delta S}_{M}^{max})\), where \({\Delta S}_{M}^{max}\) has been defined in terms of the maximum change occurring in magnetic entropy over various applied fields versus rescaled temperature (θ), this is realized at the transitions of magnetic order that are plotted in Fig. 14. The temperature axis below and above TCcan be rescaled in a very different way using the equation given below:
Considering that, Tr1 and Tr2 have been defined as the two-reference point’s temperatures that have been selected as correspondents to \({\Delta S}_{M}^{max}/2\).
In our case, over a very wide range of temperatures, there is only one universal curve for all the experimental points. This reveals that a second-order phase transition is observed for all the samples investigated in this work.
4 Conclusion
In summary, physical properties involving the structure, the magnetic behavior and some magnetocaloric parameters of \({\text{La}}_{{0.67}} {\text{Ca}}_{{0.33 - x}} {\text{Sr}}_{x} {\text{Mn}}_{{0.98}} {\text{Ni}}_{{0.02}} {\text{O}}_{3}\) (x = 0.15, 0.2, and 0.3) samples have been examined. All samples are processed using the sol–gel method and crystallize in the rhombohedral structure with \(R\bar{3}c\) space group. All compounds indicated a ferromagnetic–paramagnetic phase transition. Furthermore, the Curie temperature TC increases from 319 to 353 K when x increases from 0.15 to 0.3.
Based on Maxwell's thermodynamic relations, the magnetic entropy change (− ΔSM) has been computed and then determined using Landau's theory. A very good agreement between both analyses confirmed that electron interaction and magnetoelastic coupling of electrons are very important in magnetocaloric properties of manganites. A second-order kind of the magnetic phase transition has been confirmed using the phenomenological universal curve of ΔSM. Finally, this study has confirmed that all compounds could be regarded as good candidates for use in magnetic refrigeration applications, given the high entropy values that are comparable to those of other manganites.
References
J.M.D. Coey, M. Viret, S. von Molnar, Mixed-valence manganites. Adv. Phys. 48, 167–293 (1999)
Y. Motome, N. Furukawa, Disorder effect on spin excitation in double-exchange systems. Phys. Rev. B 71, 014446 (2005)
K. Laajimi, M. Khlifi, E.K. Hlil, K. Taibi, M.H. Gazzah, J. Dhahri, Room temperature magnetocaloric effect and critical behavior in La0.67Ca0.23Sr0.1Mn0.98Ni0.02O3 oxide. J. Mater. Sci.: Mater. Electron. 30, 11868–11877 (2019)
I. Walha, E. Dhahri, E.K. Hlil, Magnetocaloric effect and its correlation with critical behavior in La0.5Ca0.4Te0.1MnO3 manganese oxide. J. Alloys Compd. 680, 169–176 (2016)
H. Walha, H. Ehrenberg, A.C. Fuess, Structural and magnetic properties of La0.6–xCa0.4MnO3 (0 ≤ x ≤ 0.2) perovskite manganite. J. Alloys Compd. 485, 64–68 (2009)
S. Jin, T.H. Tiefel, M. McCormack, R.A. Fastnacht, R. Ramesh, L.H. Chen, Science 264, 413–415 (1994)
K. Chahara, T. Ohno, M. Kasai, Y. Kozono, Magnetoresistance in magnetic manganese oxide with intrinsic antiferromagnetic spin structure. Appl. Phys. Lett. 63, 1990 (1993)
K.A. Gschneidner, V.K. Pecharsky, A.O. Tsokol, Recent developments in magnetocaloric materials. Rep. Prog. Phys. 68, 1479 (2005)
Z.A. Mohamed, E. Tka, J. Dhahri, E.K. Hlil, Giant magnetic entropy change in manganese perovskite La0.67Sr0.16Ca0.17MnO3 near room temperature. J. Alloys Compd. 615, 290–297 (2014)
S. Mnefgui, A. Dhahri, N. Dhahri, E.L.K. Hlil, J. Dhahri, The effect deficient of strontium on structural magnetic and magnetocaloric properties of La0.57Nd0.1Sr0.33-xMnO3 (x=0.1 and 0.15) manganite. J. Magn. Magn. Mater. 340, 91–96 (2013)
T. Tang, Q.Q. Cao, K.M. Gu, H.Y. Xu, S.Y. Zhang, Y.W. Du, Giant magnetoresistance of the La1−xAgxMnO3La1−xAgxMnO3 polycrystalline inhomogeneous granular system. Appl. Phys. Lett. 77, 723 (2000)
T. Tang, K.M. Gu, Q.Q. Cao, D.H. Wang, S.Y. Zhang, Y.W. Du, Magnetocaloric properties of Ag-substituted perovskite-type manganites. J. Magn. Magn. Mater. 222, 110–114 (2000)
K. Laajimi, M. Khlifi, E.K. Hlil, M.H. Gazzah, J. Dhahri, Enhancement of magnetocaloric effect by Nickel substitution in La0.67Ca0.33Mn0.98Ni0.02O3 manganite oxide. J. Magn. Magn. Mater. 491, 165625 (2019)
G.J. Owens, R.K. Singh, F. Foroutan, M. Alqaysi, C.-M. Han, C. Mahapatra, H.-W. Kim, J.C. Knowles, Sol–gel-based materials for biomedical applications. Prog. Mater. Sci. 77, 1–79 (2016)
Y. Luo, Z. Xia, Effect of partial nitridation on the structure and luminescence properties of melilite-type Ca2Al2SiO7:Eu2+ phosphor. Opt. Mater. 36, 1874–1878 (2014)
J. Kacher, C. Landon, B.L. Adams, D. Fullwood, Bragg's Law diffraction simulations for electron backscatter diffraction analysis. Ultramicroscopy 109, 1148–1156 (2009)
M. Nasri, M. Triki, E. Dhahri, E.K. Hlil, Critical behavior in Sr-doped manganites La0.6Ca0.4−xSrxMnO3. J. Alloys Compd. 546, 84–91 (2013)
A.R. Dinesen, S. Linderoth, S. Mørup, Direct and indirect measurement of the magnetocaloric effect in La0.67Ca0.33−xSrxMnO3±δ (). J. Phys. Condens. Mater. 17, 6257 (2005)
A. Guinier, Théorie et Technique de la radiocristallographie, 3rd edn. (Editions Dunod, Paris, 1964), p. 462
S. Das, T.K. Dey, Structural and magnetocaloric properties of La1−yNayMnO3 compounds prepared by microwave processing. J. Phys. D 40, 1855–1863 (2007)
V. Barsan, Inverses of Langevin, Brillouin and related functions: a status report. Roman. Rep. Phys. 72, 109 (2020)
M. Stier, A. Neumann, A. Philippi-Kobs, H.P. Oepen, M. Thorwart, Implications of a temperature-dependent magnetic anisotropy for superparamagnetic switchin. J. Magn. Magn. Mater. 447, 96–100 (2018)
M. Nasri, M. Triki, E. Dhahri, M. Hussein, P. Lachkar, E.K. Hlil, Structural and magnetocaloric properties of La1− yNayMnO3 compounds prepared by microwave processing. Phys. B 408, 104–109 (2013)
E.L. Hernandez-Gonzalez, B.E. Watts, S.A. Palomares-Sanchez, J.T. Elizaldalindo, M. Mirabal-Garcia, Second-order magnetic transition in La0.67Ca0.33−xSrxMnO3 (x = 0.05 0.06 0.07 0.08). J. Supercond. Nov. Magn. 29, 2421–2427 (2016)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 32, 751–767 (1976)
A. Tozri, M. Bejar, E. Dhahri, E.L. Hlil, Structural and magnetic characterisation of the perovskite oxides La0.7Ca0.3−xNaxMnO3. Open Phys. 7, 89 (2009)
I. Berenov, F. Le Goupil, N. Alford, Effect of ionic radii on the Curie temperature in Ba1-x-ySrxCayTiO3 compounds. Sci. Rep. 6, 28055 (2016)
D. Sinclair, J. Paul Attfield, The influence of A-cation disorder on the Curie temperature of ferroelectric ATiO3 perovskites. Chem. Commun. 16, 1497 (1999)
S. Hcini, M. Boudard, S. Zemni, Study of Na substitution in La0.67Ba0.33MnO3 perovskites. Appl. Phys. A 115, 985–996 (2014)
B.K. Banerjee, On a generalised approach to first and second order magnetic transitions. Phys. Lett. 12, 16–17 (1964)
S.S. Nair, M. Mathews, P.A. Joy, S.D. Kulkarni, M.R. Anantharaman, Effect of cobalt doping on the magnetic properties of superparamagnetic γ-Fe2O3-polystyrene nanocomposites. J. Magn. Magn. Mater. 283, 344–352 (2004)
S.J. Haralkar, R.H. Kadam, S.S. More, S.E. Shirsath, M.L. Mane, S. Patil, D.R. Mane, Substitutional effect of Cr3+ ions on the properties of Mg–Zn ferrite nanoparticles. Phys. B 407, 4338–4346 (2012)
S. Thankachan, B.P. Jacob, S. Xavier, E.M. Mohammed, Effect of neodymium substitution on structural and magnetic properties of magnesium ferrite nanoparticles. Phys. Scr. 87, 025701 (2013)
M.S. Anwar, I. Hussain, B.H. Koo, Reversible magnetocaloric response in Sr2FeMoO6 double pervoskite. Mater. Lett. 181, 56–58 (2016)
M. Oumezzine, S. Zemni, O. Peña, Room temperature magnetic and magnetocaloric properties of La0.67Ba0.33Mn0.98Ti0.02O3 perovskite. J. Alloys Compd. 508, 292–296 (2010)
S.E.L. Kossi, S. Ghodhbane, S. Mnefgui, J. Dhahri, E.K. Hlil, The impact of disorder on magnetocaloric properties in Ti-doped manganites of La0.7Sr0.25Na0.05Mn(1–x)TixO3 (0≤x≤0.2). J. Magn. Magn. Mater. 395, 134–142 (2015)
D.T. Morelli, A.M. Mance, J.V. Mantese, A.L. Micheli, Magnetocaloric properties of doped lanthanum manganite films. J. Appl. Phys. 79, 373 (1996)
V.K. Pecharsky, K.A. Gschneidner Jr., Magnetocaloric effect from indirect measurements: magnetization and heat capacity. J. Appl. Phys. 86, 565 (1999)
E.L.T. França, A.O. dos Santos, A.A. Coelho, L.M. da Silva, Magnetocaloric effect of the ternary Dy, Ho and Er platinum gallides. J. Magn. Magn. Mater. 401, 1088–1092 (2016)
J.S. Amaral, M.S. Reis, V.S. Amaral, T.M. Mendonca, J.P. Araujo, M.A. Sa, P.B. Tavares, J.M. Vieira, Magnetocaloric effect in Er- and Eu-substituted ferromagnetic La-Srmanganites. J. Magn. Magn. Mater. 290–291, 686–689 (2005)
V.S. Amaral, J.S. Amaral, Magnetoelastic coupling influence on the magnetocaloric effect in ferromagnetic materials, magnetoelastic coupling influence on the magnetocaloric effect in ferromagnetic materials. J. Magn. Magn. Mater. 272–276, 2104–2105 (2004)
R. Hamdi, A. Tozri, E. Dhahri, L. Bessais, Structural, magnetic, magnetocaloric and electrical studies of Dy0.5(Sr1−xCax)0.5MnO3 manganites. J. Magn. Magn. Mater. 444, 270–279 (2017)
M. Foldeaki, R. Chahine, B.R. Gopal, T.K. Bose, Investigation of the magnetic properties of the Gd1−xErx alloy system in the x < 0.62 composition range. J. Magn. Magn. Mater. 150, 421–429 (1995)
D.N.H. Nam, N.V. Dai, L.V. Hong, N.X. Phuc, S.C. Yu, M. Tachibana, E. Takayama Muromachi, Room-temperature magnetocaloric effect in La0.7Sr0.3Mn1−xM′xO3 (M′=Al, Ti). J. Appl. Phys. 103, 043905 (2008)
V. Franco, J.S. Blázquez, A. Conde, Influence of Ge addition on the magnetocaloric effect of a Co-containing Nanoperm-type alloy. J. Appl. Phys. 103, 07B316 (2008)
Acknowledgement
The authors extend their appreciation to the deanship of Scientific Research at Majmaah University for funding this work under Project No (RGP-2019-12).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laajimi, K., Khlifi, M., Hlil, E.K. et al. Influence of Sr substitution on structural, magnetic and magnetocaloric properties in La0.67Ca0.33−xSrxMn0.98Ni0.02O3 manganites. J Mater Sci: Mater Electron 31, 15322–15335 (2020). https://doi.org/10.1007/s10854-020-04096-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04096-x