Abstract
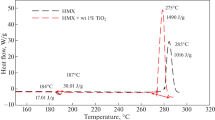
Even though HMX is one of the most powerful highly explosive materials; HMX-based propellants demonstrated complexity of burning rate control as well as high pressure exponent (n). In addition, HMX is insensitive to common catalyst. TiO2 can offer novel catalyzing ability for HMX. Highly-crystalline, mono-dispersed TiO2 NPs of 5.0 nm particle size with proper surface area (26.87 ± 0. 36 m2/g) were fabricated using hydrothermal processing. TiO2 NPs were re-dispersed in organic solvent and effectively-integrated into HMX via co-precipitation technique; the impact of TiO2 NPs on HMX thermal behavior was investigated using DSC and TGA. TiO2 NPs exposed superior catalytic performance; the endothermic phase change of HMX at 187 °C was decreased by 43.3%. The main exothermic decomposition peak was decreased by 10 °C with enhanced total heat release by 46.7%. The catalytic performance of TiO2 NPs could be ascribed to the release of active surface ȮH radicals that could induce HMX decomposition via hydrogen abstraction. Furthermore, TiO2 NPs could adsorb evolved NO2 on its surface with surge in total heat release in condensed phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
HMX is one of the most vigorous explosive materials in terms of heat output and gaseous products [1, 2]. HMX can offer large volume of gaseous products at low molecular weight [3,4,5,6]. Therefore, HMX has found wide applications in solid rocket propellant [7,8,9]. It was reported that HMX is insensitive to traditional catalysts [10]. The main approach that could affect thermolysis of HMX includes hydrogen atom abstraction with heterocyclic ring cleavage [11, 12].
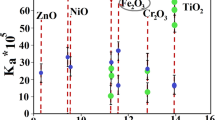
Transition metal oxides were reported to have catalytic influence on HMX thermolysis with decrease in its onset decomposition temperature [7, 13]. Nanopowders, with increased surface areas, are promising materials for catalytic applications of different energetic systems [14]. High catalytic effect on HMX was reported through TiO2 NPs. HMX catalyzation includes a decrease in onset decomposition temperature, high reaction rate, and decrease in pressure exponent value [15,16,17]. These effects could be achieved using TiO2 NPs. Enhanced catalytic performance could be accomplished with particle size decrease [18,19,20].
Some metal oxide nanocomposites were prepared by different green methods which are used for the catalytic performance and other critical applications. Sol–gel, sol–gel-hydrothermal, and photo-deposition methods were used to synthesize pure TiO2, PdO/TiO2, and Pd/TiO2 nanostructures. Citric acid was used as a stabilizer, reducing, and capping agent, since it is green, available, clean, and nontoxic [21].
A study conducted by Safajou et al. [22] shows that TiO2 nanowire (NWs) was prepared by an alkaline hydrothermal process and the following formula Graphene/Pd/TiO2 NPs and Graphene/Pd/TiO2-NWs were synthesized by a combination of hydrothermal and photo-deposition methods. The synthesized nanocomposites were investigated for their enhanced photocatalytic degradation of dyes.
Also, photo-degradation of methylene blue was investigated by the utilization of the mixed metal oxides such as Fe2O3–TiO2 NPs, TiO2@SiO2 core/shell NPs and N-doped graphene quantum dot/TiO2 nanocomposite [23,24,25]. Finally, the photocatalytic degradation of azo dyes using TiO2 NPs supported Ag NPs which prepared by a green method was investigated by Rostami-Vartooni et al. [26].
Different chemical methods such as successive ion layer adsorption and reaction, chemical bath deposition, microwave, and hydrothermal served to deposition of CdS on the prepared TiO2 surface for use in different optoelectronic fields. TiO2/CdS nanocomposite was synthesized by hydrothermal method and was then deposited on the FTO surface to investigate their influences on the dye-sensitized solar cell performance [27]. Also, TiO2 NPs were prepared using tripodal tetra-amine ligands (complexing agent) by two-step sol–gel method for the application in dye-sensitized solar cells [28]. The effect of the ligand on the synthesis of different metal oxide NPs must be taken into consideration, and the size and optical properties of TiO2 NPs in a two-step sol–gel method was altered after the use of Schiff base ligands [29]. Also, the effect of tertiary amines on the synthesis and photovoltaic properties of TiO2 NPs in dye-sensitized solar cells was investigated [30]. Finally, a stable plasmonic-improved dye-sensitized solar cells was achieved by Ag NPs between TiO2 Layers [31].
It must be noted that, the as-fabricated mesoporous TiO2 fibers exhibit much higher photocatalytic activity and stability than both the conventional solid counterparts and the commercially-available P25. The abundant vapors released from the introduced foaming agents are responsible for the creation of pores with uniform spatial distribution in the spun precursor fibers [32]. In another study regarding nanomaterials-based TiO2 NPs, a novel and highly efficient visible-light-driven photocatalyst with robust stability made up of thoroughly mesoporous TiO2/WO3/g-C3N4 ternary hybrid nanofibers and TiO2/CuO/Cu had been fabricated through a foaming-assisted electro-spinning process followed by a solution-dipping process [33, 34]. Finally, a brilliant BiVO4@TiO2 core–shell hybrid mesoporous nanofiber was used for efficient visible-light-driven photocatalytic hydrogen production [35].
1.1 TiO2 catalyzation mechanism
It is widely established that nitramine decomposition can be catalyzed with ȮH radicals. TiO2 NPs are characterized with hydrous surface (surface-bound hydroxyl groups). The release of surface ȮH radicals can speed up HMX decomposition [36]. The required activation energy to liberate ȮH radicals from TiO2 surface is 65 kJ/mol; this value could decrease with the increase in the particle surface area [16]. Furthermore, NPs surface could absorb gaseous products offering high heat output [20]. TiO2 NPs could lower the required activation energy for HMX decomposition. Whereas HMX normal decomposition process include C-N bond breakage of heterocyclic ring, decomposition of catalyzed HMX with nanocatalyst includes dehydration of catalyst surface with the release of active ȮH radicals; these radicals would abstract H-atom from the heterocyclic ring [37].
Therefore, the catalytic decomposition process could take place at lower temperature and with lower activation energy [38]. The strength of surface-bounded –OH groups is a key parameter for the catalytic activity of oxides. Electronegativity of metal cation xi expresses the capability to withdraw electron pair (Eq. 1).
where xo and n are electronegativity of metal atom and the metal charge in the oxide state, respectively.
Metal oxide with high Xi have acid properties, whereas oxides with low Xi have base properties. Oxide point of zero charge (isoelectric point) describes the surface acidity; it is equal to the medium acidity in which oxide surface has no electric charge.
TiO2 NPs were verified to have superior efficiency compared with other oxides as well as microsize TiO2 [39, 40]. Reliable fabrication of nanoscopic TiO2 is an urgent demand. There is a vast benefit for synthesis technology that could offer fabrication of TiO2 NPs with constant product quality. Hydrothermal processing offered consistent fabrication of different oxide particles in dispersion [41].
1.2 Hydrothermal processing
Hydrothermal processing was reported to be a beneficial technology that could offer fabrication of highly crystalline oxides in dispersion [42, 43]. This technology includes direct mixing of metal salt feed with supercritical fluid (ScF),ScF can expose distinctive characteristics in terms of enhanced levels of OH− [18, 44,45,46,47,48]. Above critical conditions, phase boundary vanishes and a homogenous supercritical phase exists as displayed in Fig. 1 [49, 50].
Phase boundary of ScF with temperature and pressure [51]
Oxide fabrication can be achieved via hydrolysis of metal salt with subsequent dehydration step (Eqs. 2 and 3) [52,53,54].
Accordingly, this study reports on the consistent fabrication of TiO2 NPs of 5 nm particle size using hydrothermal synthesis. TiO2 NPs were developed in dispersion; consequently, colloidal TiO2 NPs were integrated into HMX particles. Uniform dispersion of TiO2 NPs into HMX was verified using SEM/mapping technique. The effectiveness of TiO2 NPs on HMX thermal decomposition was investigated using DSC and TGA. TiO2 NPs demonstrated superior catalytic efficiency. The endothermic phase change at 187 °C was decreased by 43.3%. The exothermic decomposition temperature was decreased by 10 °C with an increase in total heat release by 46.7%. This superior catalytic performance was accomplished at 1 wt % catalyst. TiO2 NPs catalyzing mechanism was correlated to the release of active surface ȮH radicals that could attack the heterocyclic ring with hydrogen atom abstraction with heterocyclic ring cleavage.
2 Experimental work
2.1 Hydrothermal synthesis of TiO2 NPs
The employed metal salt for TiO2 NP synthesis was titanium (IV) bis (ammonium lactato) dihydroxide ([CH3CH(O–)CO2NH4]2Ti(OH)2) (TIBALD) 50 wt% in H2O solution (CAS number 65104-06-5, Aldrich, Germany). ScW was employed at 400 °C, 240 bars (20 ml/min) (Flow A). 0.05 M solution of TIBALD in deionized water was employed at 25 °C, 240 bars (10 ml/min) (Flow B). TiO2 NPs were developed at the boundary of the flow (Fig. 2). Further details regarding the hydrothermal synthesis of TiO2 NPs can be observed in the following references [55,56,57,58].
2.2 Characterization of TiO2 NPs
Crystallinity and phase were investigated using X-ray diffraction (XRD) spectroscopy on a Brucker axis D8 diffractometer applying radiation of Cu Kα with (λ = 1.540598 Å), voltage of 40 kV, and current of 40 mA. The average nanostructure and the particle size determination of the synthesized TiO2 NPs were determined by applying a High-Resolution Transmission Electron Microscope (HRTEM, JEM2100, Jeol, Japan). The surface morphology and a specific appearance of the dry particles (pure TiO2 NPs) were examined with Scanning Electron Microscope (SEM, ZEISS, EVO-MA10, Germany). On the other hand, EDX technique (BRUKER, Nano GmbH, D-12489, 410-M, Germany) was applied to investigate the elemental configuration and the atomic percentage of the metals detected in the prepared samples. FTIR spectrometer Nicolet 380 by Thermo-electron Corporation was employed to investigate the nanoparticle chemical structure and their functional groups. Brunauer–Emmett–Teller (BET) method was used to describe the surface area and the measurements were carried out via the surface area analyzer (Nova 3200 Nitrogen Physisorption Apparatus USA) with liquid N2 as an adsorbate at − 196 °C. Finally, the mapping analysis after applied SEM/EDX technique was used to attain whole information about the clarity, distribution, and the position of the metals (pure TiO2 NPs) on the surface of HMX.
2.3 Integration of TiO2 NPs into HMX
All classical NP synthesis techniques include sintering and drying process which result in a dramatic decrease in NP surface area and reactivity. This is the first time ever to report on fabrication of colloidal TiO2 NPs and their integration into HMX crystalline structure. This approach could offer extensive surface area and reactivity; it could eliminate NP drying and the re-dispersion of dry aggregates.
TiO2 NPs were decanted from their synthesis medium and re-dispersed in acetone using ultrasonic probe homogenizer. HMX was dissolved in acetone colloid. The ratio of TiO2 NPs: HMX was 1: 99. TiO2 NPs were integrated into HMX using co-precipitation technique. The size and shape of TiO2/HMX hybrid was investigated using SEM/EDX mapping technique for giving further information regarding the simplicity, relationships, and the position of the TiO2 NPs incorporated with HMX.
2.4 Thermal behavior of catalyzed HMX
Thermal behavior of HMX catalyzed with TiO2 NPs was investigated using DSC Q20 by TA. Tested sample was heated from 50 to 500 °C. The heating rate was 5 °C/min, under N2 flow of 50 ml/min. The impact of TiO2 NPs on HMX weight loss was evaluated using TGA 55 by TA. The tested sample was heated from 50 to 500 °C. The heating rate was 5 °C/min under N2 flow at 25 ml/min.
3 Result and discussions
3.1 Characterization of the synthesized TiO2 NPs
TEM micrographs of the synthesized TiO2 NPs demonstrated mono-dispersed particles with uniform particle size and the particle size was found to be ranging from 3.0 to 10.0 nm with an average particle size recorded at 5.0 nm (Fig. 3a). HRTEM images provided a detailed investigation of structure, shape, and size of TiO2 NPs which demonstrated a high crystalline structure with spherical and cubic structures (Fig. 3b, c). This result was matched with the results described in previous publications [59,60,61].
Figure 4 confirmed high-quality mono-dispersed particles and high crystalline structure. The crystalline structure was investigated with X-ray diffraction (XRD). XRD pattern confirmed high-quality anatase crystalline structure (Fig. 4); this is the most common crystalline structure in catalyst applications. For data analysis in Fig. 4, sharp, strong, and intense peaks are observed in 2Ɵ = 25.1° (101), 28.1° (110), 37.5° (004), 48.9° (200), 54.4° (105), 55.6° (211), and 62.8° (204), while the main peak is located at 2Ɵ = 25.4°, these peaks are in a good matching with those of reference anatase TiO2 NPs (JCPDS 04-0477) [62]. This result was matched with the results described in previous publications [63,64,65,66]. The average crystallite size was calculated using the Debye–Scherrer Eq. (4) [67] and was found to be 10.12 nm:
where K = 0.9 and known as shape factor, λ is x-rays’ wavelength (1.54060 Ǻ for Cu-Kα), β is full width at half maximum (FWHM), and θ is the diffraction angle.
SEM image of the fabricated TiO2 NPs is presented in Fig. 5 a; the synthesized TiO2 layer appears as a uniform and bright layer, also, the corresponding EDX analysis (Fig. 5b) was similar in words of diffusion (Ti, O, and C atoms) over the grain lines. Also, the carbon atoms were due to the holder which is used in the imaging process [68]. This result was matched with the results described in previous publications [69,70,71,72].
FTIR spectrum was a significant study that provides important data about the chemical functional groups represented in TiO2 NPs [73]. FTIR spectrum of developed TiO2 NPs confirmed the hydrous surface. The enhanced levels of IR absorption at 3500 cm−1 can be correlated to the O–H surface group stretch as shown in Fig. 6. The antatase Titania appears at the region from 800 to 400 cm−1 [74]. This result was matched with the results described in previous publications [75,76,77].
The electrical and chemical properties are dependent on the specific surface area and grain size, as the chemical and physical phenomena controlled by surface porosity and electrons conduction occur at TiO2 NP’s surface [78]. N2 adsorption–desorption isotherm of the prepared TiO2 NPs is shown in Fig. 7.
According to the IUPAC classification, the obtained isotherm was of type (IV), indicating the presence of mesopores. The uptake of adsorbate was increased when pores became filled, and an inflection point occurred near the completion of the first monolayer [62, 77]. From Fig. 7, the calculated surface area of the prepared TiO2 NPs was 26.87 ± 0. 36 m2/g, a similar behavior was detected in the literature and matched our BET result [77, 79,80,81].
Morphology of TiO2-HMX nanocomposite was investigated with SEM, to verify the uniform integration of TiO2 NPs into HMX crystalline structure [82], while EDX examination was performed for its elemental analysis and purity estimation [83,84,85].
Dry agglomerates include drastic decrease in surface area and reactivity; therefore, the particles would act as micron rather than NPs [44, 54]. Consequently, integration of colloidal particles into HMX could maintain high surface area and reactivity.
Elemental mapping using SEM revealed uniform dispersion of TiO2 NPs into HMX as shown in Fig. 8. Co-precipitation technique offered uniform dispersion of TiO2 NPs into HMX. This approach could offer superior interfacial surface area (the calculated surface area of the prepared TiO2 NPs was 26.87 ± 0. 36 m2/g; Fig. 7) and catalytic performance. This result was matched with the results described in previous publications [66, 86,87,88,89].
3.2 Catalytic activity of TiO2 NPs
TiO2 NPs demonstrated dramatic change in HMX thermal behavior. The endothermic phase change of HMX at 187 °C was decreased by 43.3%. The main outcome of this study is that temperature at maximum heat release rate was decreased by 10 °C with an increase in total heat release rate by 46.7% as exhibited in Fig. 9.
The catalytic activity of TiO2 NPs was further evaluated with TGA. TGA thermogram confirmed DSC outcomes; temperature at total weight loss was decreased by 10 °C as displayed in Fig. 10a and b.
At temperature higher than 150 °C, ȮH radicals would be evolved from TiO2 NPs surface. These active radicals will have high ability to abstract hydrogen from HMX structure [90]. After hydrogen abstraction, energy of N-NO2 bond would decrease significantly; this could lead to release of nitro group (NO2) [91]. The evolved NO2 group could abstract another H-atom from another HMX molecule. Adsorption of NO2 on the surface of TiO2 could increase the heat release in condensed phase as shown in Fig. 11 [7].
Whereas CH2O and N2O fragment will be evolved at low heating rate; HCN and NO2 will be evolved at high heating rate. Moreover, CH2O could be due to ȮH interaction with double bond. The main TiO2 NPs catalytic steps include de-hydroxylation of the metal oxide surface with the release of active ȮH radicals, nitramine decomposition through hydrogen abstraction with ȮH radicals, and adsorption of liberated NO2 on the surface of TiO2 NPs. At high decomposition temperature, the reaction of CH2O and NO2 would provide the main exothermic reaction.
4 Conclusion
Hydrothermal processing was reported to be a beneficial technology that could offer fabrication of highly-crystalline TiO2 NPs in dispersion. The particle size of the synthesized TiO2 NPs was found to be ranging from 3.0 to 10.0 nm with an average particle size recorded at 5.0 nm, and the calculated surface area of the prepared TiO2 NPs was found to be 26.87 ± 0.36 m2/g. The effective coating of TiO2 with HMX was conducted via co-precipitation technique. The synthesized TiO2 NPs demonstrated superior catalytic activity on HMX thermolysis. TiO2 NPs demonstrated dramatic change in HMX thermal behavior. The endothermic phase change of HMX at 187 °C was decreased by 43.3%. The main outcome of this study is that temperature at maximum heat release rate was decreased by 10 °C with an increase in total heat release rate by 46.7%. At temperature higher than 150 °C, ȮH radicals would be evolved from TiO2 NPs surface. These active radicals will have high ability to abstract hydrogen from HMX structure. TiO2 NPs catalytic mechanism includes the following: (1) Release of ȮH radicals initiating destruction of HMX molecule and (2) Adsorption of released NO2 to the NPs surface. Therefore, the total heat release would increase significantly. Integration of colloidal TiO2 NPs into HMX would secure high reactivity.
References
M. Meyer, J. Kohler, A. Homburg (eds.), Explosives, 6th edn. (Wiley, Weinheim, 2007)
F. Monteil-Rivera et al., Dissolution of a new explosive formulation containing TNT and HMX: comparison with octol. J. Hazard. Mater. 174(1), 281–288 (2010)
N. Kubota (ed.), Propellants and Explosives Thermochemical Aspects of Combustion (Wiley-VCH, Weinheim, 2002)
S. Elbasuney, A. Fahd, H.E. Mostafa, Combustion characteristics of extruded double base propellant based on ammonium perchlorate/aluminum binary mixture. Fuel 208(Supplement C), 296–304 (2017)
S. Elbasuney et al., Chemical stability, thermal behavior, and shelf life assessment of extruded modified double-base propellants. Def. Technol. 14(1), 70–76 (2018)
E.S. Kim, V. Yang, Y.C. Liau, Modeling of HMX/GAP pseudo-propellant combustion. Combust. Flame 131(3), 227–245 (2002)
V.E. Zarko, A.A. Gromov (eds.), Energetic Nanomaterials Synthesis, Characterization, and Application (Elsevier, Amsterdam, 2016)
Q.-L. Yan et al., Combustion mechanism of double-base propellant containing nitrogen heterocyclic nitroamines (I): the effect of heat and mass transfer to the burning characteristics. Combust. Flame 156(3), 633–641 (2009)
K.V. Meredith, M.L. Gross, M.W. Beckstead, Laser-induced ignition modeling of HMX. Combust. Flame 162(2), 506–515 (2015)
L. Patidar, M. Khichar, S.T. Thynell, Identification of initial decomposition reactions in liquid-phase HMX using quantum mechanics calculations. Combust. Flame 188, 170–179 (2018)
Q.-L. Yan et al., Catalytic effects of nano additives on decomposition and combustion of RDX-, HMX-, and AP-based energetic compositions. Prog. Energy Combust. Sci. 57, 75–136 (2016)
R. Liu, P.W. Chen, Modeling ignition prediction of HMX-based polymer bonded explosives under low velocity impact. Mech. Mater. 124, 106–117 (2018)
A. Singh et al., Thermal decomposition and kinetics of plastic bonded explosives based on mixture of HMX and TATB with polymer matrices. Def. Technol. 13(1), 22–32 (2017)
X.-G. Wu et al., Combustion efficiency and pyrochemical properties of micron-sized metal particles as the components of modified double-base propellant. Acta Astronaut. 68(7), 1098–1112 (2011)
J.J. Xiao et al., Study on structure, sensitivity and mechanical properties of HMX and HMX-based PBXs with molecular dynamics simulation. Comput. Theor. Chem. 999, 21–27 (2012)
A.N. Pivkina et al., Chapter nine—catalysis of HMX decomposition and combustion: defect chemistry approach, in Energetic Nanomaterials, ed. by V.E. Zarko, A.A. Gromov (Elsevier, Amsterdam, 2016), pp. 193–230
Y. Wang et al., Combustion synthesis of La0.8Sr0.2MnO3 and its effect on HMX thermal decomposition. Chin. J. Chem. Eng. 18(3), 397–401 (2010)
S. Elbasuney, Sustainable steric stabilization of colloidal titania nanoparticles. Appl. Surf. Sci. 409, 438–447 (2017)
M.A. Elsayed, M. Gobara, S. Elbasuney, Instant synthesis of bespoke nanoscopic photocatalysts with enhanced surface area and photocatalytic activity for wastewater treatment. J. Photochem. Photobiol. A 344, 121–133 (2017)
R. Liu et al., Dynamic vacuum stability test method and investigation on vacuum thermal decomposition of HMX and CL-20. Thermochim. Acta 537, 13–19 (2012)
H. Khojasteh et al., Synthesis, characterization and photocatalytic activity of PdO/TiO2 and Pd/TiO2 nanocomposites. J. Mater. Sci.: Mater. Electron. 27(2), 1261–1269 (2016)
H. Safajou et al., Enhanced photocatalytic degradation of dyes over graphene/Pd/TiO2 nanocomposites: TiO2 nanowires versus TiO2 nanoparticles. J. Colloid Interface Sci. 498, 423–432 (2017)
A. Abbasi et al., Photo-degradation of methylene blue: photocatalyst and magnetic investigation of Fe2O3–TiO2 nanoparticles and nanocomposites. J. Mater. Sci.: Mater. Electron. 27(5), 4800–4809 (2016)
T. Gholami et al., Photocatalytic degradation of methylene blue on TiO2@SiO2 core/shell nanoparticles: synthesis and characterization. J. Mater. Sci.: Mater. Electron. 26(8), 6170–6177 (2015)
H. Safardoust-Hojaghan, M. Salavati-Niasari, Degradation of methylene blue as a pollutant with N-doped graphene quantum dot/titanium dioxide nanocomposite. J. Clean. Prod. 148, 31–36 (2017)
A. Rostami-Vartooni et al., Photocatalytic degradation of azo dyes by titanium dioxide supported silver nanoparticles prepared by a green method using Carpobrotus acinaciformis extract. J. Alloys Compd. 689, 15–20 (2016)
M. Sabet, M. Salavati-Niasari, O. Amiri, Using different chemical methods for deposition of CdS on TiO2 surface and investigation of their influences on the dye-sensitized solar cell performance. Electrochim. Acta 117, 504–520 (2014)
N. Mir, M. Salavati-Niasari, Preparation of TiO2 nanoparticles by using tripodal tetraamine ligands as complexing agent via two-step sol–gel method and their application in dye-sensitized solar cells. Mater. Res. Bull. 48(4), 1660–1667 (2013)
M. Masjedi et al., Effect of Schiff base ligand on the size and the optical properties of TiO2 nanoparticles. Superlattices Microstruct. 62, 30–38 (2013)
N. Mir, M. Salavati-Niasari, Effect of tertiary amines on the synthesis and photovoltaic properties of TiO2 nanoparticles in dye sensitized solar cells. Electrochim. Acta 102, 274–281 (2013)
O. Amiri et al., Stable plasmonic-improved dye sensitized solar cells by silver nanoparticles between titanium dioxide layers. Electrochim. Acta 152, 101–107 (2015)
H. Hou et al., General strategy for fabricating thoroughly mesoporous nanofibers. J. Am. Chem. Soc. 136(48), 16716–16719 (2014)
H. Hou et al., Superior thoroughly mesoporous ternary hybrid photocatalysts of TiO2/WO3/gC3N4 nanofibers for visible-light-driven hydrogen evolution. J. Mater. Chem. A 4(17), 6276–6281 (2016)
H. Hou et al., Highly efficient photocatalytic hydrogen evolution in ternary hybrid TiO2/CuO/Cu thoroughly mesoporous nanofibers. ACS Appl. Mater. Interfaces 8(31), 20128–20137 (2016)
H. Hou et al., BiVO4@TiO2 core–shell hybrid mesoporous nanofibers towards efficient visible-light-driven photocatalytic hydrogen production. J. Mater. Chem. C 7(26), 7858–7864 (2019)
S. Elbasuney, M. Yehia, Ammonium perchlorate encapsulated with TiO2 nanocomposite for catalyzed combustion reactions. J. Inorg. Organomet. Polym. Mater. 29(4), 1349–1357 (2019)
Z.-X. Wei et al., Combustion synthesis and effect of LaMnO3 and LaOCl powder mixture on HMX thermal decomposition. Thermochim. Acta 499(1), 111–116 (2010)
S. Elbasuney, M. Gobara, M. Yehia, Ferrite nanoparticles: synthesis, characterization, and catalytic activity evaluation for solid rocket propulsion systems. J. Inorg. Organomet. Polym. Mater. 29(3), 721–729 (2019)
R. Dubey et al., Synthesis, characterization and catalytic behavior of Cu nanoparticles on the thermal decomposition of AP, HMX, NTO and composite solid propellants, Part 83. Thermochim. Acta 549, 102–109 (2012)
J. Wei et al., 0D Cu(II) and 1D mixed-valence Cu(I)/Cu(II) coordination compounds based on mixed ligands: syntheses, structures and catalytic thermal decomposition for HMX. Inorg. Chem. Commun. 30, 13–16 (2013)
J.-S. Lee, C.-K. Hsu, C.-L. Chang, A study on the thermal decomposition behaviors of PETN, RDX, HNS and HMX. Thermochim. Acta 392–393, 173–176 (2002)
X. Wang, Y. Li, Selected-control hydrothermal synthesis of α-and β-MnO2 single crystal nanowires. J. Am. Chem. Soc. 124(12), 2880–2881 (2002)
S. Elbasuney, M. Yehia, Thermal decomposition of ammonium perchlorate catalyzed with CuO nanoparticles. Def. Technol. 15(6), 868–874 (2019)
S. Elbasuney, Dispersion characteristics of dry and colloidal nano-titania into epoxy resin. Powder Technol. 268, 158–164 (2014)
S. Elbasuney, Surface engineering of layered double hydroxide (LDH) nanoparticles for polymer flame retardancy. Powder Technol. 277, 63–73 (2015)
S. Elbasuney, Continuous hydrothermal synthesis of AlO(OH) nanorods as a clean flame retardant agent. Particuology 22, 66–71 (2015)
S. Elbasuney, Novel multi-component flame retardant system based on nanoscopic aluminium-trihydroxide (ATH). Powder Technol. 305, 538–545 (2017)
S. Elbasuney, Novel colloidal molybdenum hydrogen bronze (MHB) for instant detection and neutralization of hazardous peroxides. TrAC Trends Anal. Chem. 102, 272–279 (2018)
P. Savage et al., Reactions at supercritical conditions: applications and fundamentals. AIChE J 41(7), 1723–1778 (1995)
K.S. Morley et al., Clean preparation on nanoparticulate metals in porous supports: a supercritical route. J. Chem. Mater. 12, 1898–1905 (2002)
H. Hobbs, Biocatalysis in 'green solvents, in Chemistry (University of Nottingham, Notttingham, 2006)
S. Elbasuney, Novel colloidal nanothermite particles (MnO2/Al) for advanced highly energetic systems. J. Inorg. Organomet. Polym. Mater. 28(5), 1793–1800 (2018)
S. Elbasuney et al., Infrared signature of novel super-thermite (Fe2O3/Mg) fluorocarbon nanocomposite for effective countermeasures of infrared seekers. J. Inorg. Organomet. Polym. Mater. 28(5), 1718–1727 (2018)
S. Elbasuney et al., Super-thermite (Al/Fe2O3) fluorocarbon nanocomposite with stimulated infrared thermal signature via extended primary combustion zones for effective countermeasures of infrared seekers. J. Inorg. Organomet. Polym. Mater. 28, 2231–2240 (2018)
A. Bouvy, A. Opstaele (eds.), Waterbrone Coating and Additives (Royal Chemical Society, London, 1995)
S. Voyutsky (ed.), Colloid Chemistry (Mir Publisher, Moscow, 1978)
R.J. Hunter (ed.), Zeta Potential in Colloid Science (Academic Press, New York, 1981)
S. Elbasuney et al., Stabilized super-thermite colloids: a new generation of advanced highly energetic materials. Appl. Surf. Sci. 419, 328–336 (2017)
J. Lee et al., Titanium dioxide nanoparticles oral exposure to pregnant rats and its distribution. Part. Fibre Toxicol 16(1), 31 (2019)
A.C.S. de la Vega et al., Nanosized titanium dioxide UV filter increases mixture toxicity when combined with parabens. Ecotoxicol. Environ. Saf. 184, 109565 (2019)
L.W. Zhang, N.A. Monteiro-Riviere, Toxicity assessment of six titanium dioxide nanoparticles in human epidermal keratinocytes. Cutan. Ocular Toxicol 38(1), 66–80 (2019)
M. Abd Elkodous et al., Layer-by-layer preparation and characterization of recyclable nanocomposite (CoxNi1−xFe2O4; X = 0.9/SiO2/TiO2). J. Mater. Sci.: Mater. Electron. 30(9), 8312–8328 (2019)
Y. Tang et al., Measurement of SnO2 nanoparticles coating on titanium dioxide nanotube arrays using grazing-incidence X-ray diffraction, in Characterization of Minerals, Metals, and Materials 2019 (Springer, Berlin, 2019), pp. 703–711
H. Fernando et al., Synthesis, characterization and antimicrobial activity of garcinol coated titanium dioxide nanoparticles, in Proceedings of Annual Scientific Sessions of Faculty of Medical Sciences (2019)
L. Guo et al., In situ generated plasmonic silver nanoparticle-sensitized amorphous titanium dioxide for ultrasensitive photoelectrochemical sensing of formaldehyde. ACS Sens. 4(10), 2724–2729 (2019)
N. El-Shafai et al., Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide nanoparticles: fabrication, characterization, DNA interaction, and antibacterial activity. RSC Adv. 9(7), 3704–3714 (2019)
H. Gao et al., The efficient biogeneration of Ag and NiO nanoparticles from VPLE and a study of the anti-diabetic properties of the extract. RSC Adv. 10(5), 3005–3012 (2020)
M.A. Maksoud et al., Incorporation of Mn2+ into cobalt ferrite via sol–gel method: insights on induced changes in the structural, thermal, dielectric, and magnetic properties. J. Sol-Gel. Sci. Technol. 90(3), 631–642 (2019)
A.W. Jatoi, I.S. Kim, Q.-Q. Ni, Cellulose acetate nanofibers embedded with AgNPs anchored TiO2 nanoparticles for long term excellent antibacterial applications. Carbohydr. Polym. 207, 640–649 (2019)
W.R. Thalgaspitiya et al., A novel, mesoporous molybdenum doped titanium dioxide/reduced graphene oxide composite as a green, highly efficient solid acid catalyst for acetalization. Dalton Trans. 49, 3786–3795 (2020)
A. Nurfadillah, M. Nasir, R.A. Lubis, Synthesis and characterization of sulfonated PVDF TiO2-natural zeolite nanocomposites membrane, in Key Engineering Materials (Trans Tech Publ, 2019)
O. Fichera et al., Characterization of water-based paints containing titanium dioxide or carbon black as manufactured nanomaterials before and after atomization. Appl. Nanosci. 9(4), 515–528 (2019)
A.I. El-Batal et al., Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Adv. 10(16), 9274–9289 (2020)
M. Srinivasan et al., Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 80, 197–202 (2019)
H. Pouran et al., Assessment of ATR-FTIR spectroscopy with multivariate analysis to investigate the binding mechanisms of Ag and TiO2 nanoparticles to Chelex®-100 or Metsorb™ for the DGT technique. Anal. Methods (2020). https://doi.org/10.1039/C9AY02458A
T. Munir et al., Impact of silver dopant on structural and optical properties of TiO2 nanoparticles. DJNB. 14(2), 279–284 (2019).
G.S. El-Sayyad et al., Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 09 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: implication for wastewater treatment. RSC Adv. 10(9), 5241–5259 (2020)
M. Maksoud et al., Controllable synthesis of Co1−xMxFe2O4 nanoparticles (M= Zn, Cu, and Mn; x= 0.0 and 0.5) by cost-effective sol-gel approach: analysis of structure, elastic, thermal, and magnetic properties. J. Mater. Sci.: Mater. Electron. (2020). https://doi.org/10.1007/s10854-020-03518-0.pdf
M. Arulprakasajothi et al., Heat transfer study of water-based nanofluids containing titanium oxide nanoparticles. Mater. Today: Proc. 2(4–5), 3648–3655 (2015)
H. Lee, S. Jin, S. Yim, Titanium oxide nanoparticle-embedded mesoporous manganese oxide microparticles for supercapacitor electrodes. J. Phys. Chem. Solids 138, 109264 (2020)
W. Sun et al., Synthesis and enhanced electrorheological properties of TS-1/titanium oxide core/shell nanocomposite. Ind. Eng. Chem. Res. 59(3), 1168–1182 (2020)
S. Elbasuney et al., Surface modified colloidal silica nanoparticles: Novel aspect for complete identification of explosive materials. Talanta 211, 120695 (2020)
F.M. Mosallam et al., Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb Pathog. 122, 108–116 (2018)
M.A. Maksoud et al., Synthesis and characterization of metals-substituted cobalt ferrite [MxCo(1−x)Fe2O4;(M= Zn, Cu and Mn; x= 0 and 0.5)] nanoparticles as antimicrobial agents and sensors for Anagrelide determination in biological samples. Mater. Sci. Eng.: C 92, 644–656 (2018)
A. Ashour et al., Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology 40, 141–151 (2018)
M. Zhang et al., What occurs in colloidal gas aphron-induced separation of titanium dioxide nanoparticles? Particle fate analysis by tracking technologies. Sci. Total Environ. 716, 137104 (2020)
J.H. Kim et al., Mussel adhesive protein-coated titanium oxide nanoparticles for effective NO removal from versatile substrates. Chem. Eng. J. 378, 122164 (2019)
Q. Wang et al., Superhydrophobic paper fabricated via nanostructured titanium dioxide-functionalized wood cellulose fibers. J. Mater. Sci. 55(16), 7084–7094 (2020)
H. Lee et al., Titanium dioxide modification with cobalt oxide nanoparticles for photocatalysis. J. Ind. Eng. Chem. 32, 259–263 (2015)
J.J.-I. Yoh et al., Test-based thermal explosion model for HMX. Proc. Combust. Inst. 31(2), 2353–2359 (2007)
C.M. Tarver, T.D. Tran, Thermal decomposition models for HMX-based plastic bonded explosives. Combust. Flame 137(1), 50–62 (2004)
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elbasuney, S., El-Sayyad, G.S. The potentials of TiO2 nanocatalyst on HMX thermolysis. J Mater Sci: Mater Electron 31, 14930–14940 (2020). https://doi.org/10.1007/s10854-020-04054-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04054-7