Abstract
A series of Zn(Ti1−xZrx)Ta2O8 (x = 0, 0.2, 0.4, 0.6, 0.8, 1) ceramics samples were synthesized by conventional solid-state reaction route. The sintering behavior, crystal structure, Raman spectroscopy, microstructure, and microwave dielectric properties were studied. The crystal structure, lattice parameters, and bonding of the ceramics were analyzed using X-ray powder diffraction and Raman spectroscopy. As Zr4+ content increasing, the structural transition followed the sequence: tri-rutile → coexistence of tri-rutile and columbite → columbite → wolframite. The microwave dielectric properties of ceramics were considerably affected by the structural transition. Compared with pure phase ZnTiTa2O8 ceramics, a small amount of Zr-substitution (x = 0.2) could significantly improve the Q × f values (from 19,852 to 44,048 GHz) and τf values (from 77.1 to − 11.9 ppm/°C) of ceramics. Typically, ZnTi0.8Zr0.2Ta2O8 (x = 0.2) ceramics exhibited an excellent microwave dielectric property of εr ~ 29.9, Q × f ~ 44,048 GHz, and τf ~ − 11.9 ppm/°C as the sintering temperature was 1300 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With rapid development of mobile phone and modern wireless communication market, microwave dielectric materials, widely used in microwave components such as filters, wave guides resonators, duplexer, and antennas, are all significantly developed. This kind of materials needs to meet higher requirements: a medium dielectric constant (εr), a high-quality factor (Q), and a temperature frequency coefficient (τf) close to zero [1, 2].
Recently, tri-rutile structured ZnTiTa2O8 ceramics belonging to M2+TiTa2O8 (M2+=Zn, Mg, Co, Ni,) system were reported to have microwave dielectric properties of εr ∼ 46.2, Q × f ∼ 36,700 GHz and τf ∼ + 74 ppm/°C [3,4,5]. However, the large τf value restricted the practical application of this ceramics, so how to adjust the τf value and improve the dielectric loss become more and more important for this kind of ceramics. Generally, there were three main methods to adjust the temperature coefficient of materials: (1) forming multiphase ceramics by using different structural ceramics with opposite τf value; (2) forming solid solution by using ion substitution. (3) changing the inclination of oxygen octahedron through structural phase transformation. Compared with the other two methods, ion substitution was the most convenient and effective way to obtain temperature-stable ceramics without deteriorating other microwave properties (εr and Q × f value) [6]. Considering the radius of Zr4+(0.72 Å) was closed to Ti4+(0.605 Å), it was possible to form solid solution with a near-zero τf value by Zr4+ substitution for Ti4+. For instance, Hsu et al. reported microwave dielectric properties of εr ~ 46, high Q × f value ~ 47,500 GHz and τf ~ − 9.5 ppm/°C were obtained for CaLa4(ZrxTi1−x)4O15 ceramics with 0.05 wt% Zr substitution [7]. For CoTiNb2O8 ceramics, Y. Zhang et al. reported a near-zero τf of 4.4 ppm/°C could be acquired by the substitution of Zr4+ to the Ti4+ position [6]. M.J. Wu et al. investigated Ni0.5(Ti0.5−xZrx)NbO4 ceramics with τf value close to zero, which was obtained by adjusting the x value of 0.25–0.3 [8]. Moreover, Zr4+ substitution for Ti4+ could also effectively adjust the τf values without detrimental effect on Q × f values in the Zn0.5Ti1−xZrxNbO4 system [9].
At present, there were few reports on the temperature coefficient regulation of ZnTiTa2O8 ceramics. Therefore, in this work, Zr4+ was chosen as a substitute ion to obtain microwave ceramics with stable temperature coefficient and excellent microwave dielectric properties. Zn(Ti1−xZrx)Ta2O8 (x = 0, 0.2, 0.4, 0.6, 0.8, 1) ceramics were prepared via traditional solid reaction method. The sintering behavior, crystal structure, Raman spectroscopy, microstructure, and microwave dielectric properties of the ceramics were studied.
2 Experimental procedure
A series of Zn(Ti1−xZrx)Ta2O8 (x = 0, 0.2, 0.4, 0.6, 0.8, 1) ceramics samples were synthesized by conventional solid-state reaction route. Oxide powders of ZnO (99.9%), TiO2 (99.9%), ZrO2 (99.9%), and Ta2O5 (99.99%) were adopted as raw materials. Initially, according to the chemical ratio, the oxide materials was mixed with ethanol in polyethylene bottles and ball milled for 4 h. Subsequently, the slurries put into an alumina crucible were dried in a drying oven at 80 °C for 2 h, then sieved through 200 mesh and calcined at 1200 °C for 4 h with a heating rate of 5 °C/min. After milling and sieving again, the resultant powders were uniaxially compacted with 5 wt% polyvinyl alcohol into pellets under the pressure of 200 MPa. Finally, the green pellets were sintered at the temperature range of 1200–1350 °C for 4 h with a heating rate of 5 °C/min.
The relative densities of sintered samples were identified by Archimedes method. Crystal structure was undertaken by X-ray diffraction (XRD, Rigaku, DMAX- RB, Japan) with Cu Kα radiation. Raman spectroscopy of these polished samples was acquired by a LabRam HR (Jobin-Yvon, France) excited with an Ar+ laser (514.5 nm line). Microstructure of these ceramics samples was observed with scanning electron microscope (SEM, JSM-6480LV). Microwave dielectric properties of the ceramics were measured by a network analyzer (8720ES, Agilent, USA) using Hakki–Coleman’s dielectric resonator method, modified and improved by Courtney and Kobayashi [10,11,12]. All measurements were taken in the frequency of 6–9 GHz at room temperature. The temperature coefficients of the resonant frequency (τf) were measured in the temperature ranging from 25 to 80 °C. The τf values were defined by the following relationship:
where f1 and f2 were the resonant frequency at T1 and T2, respectively.
3 Results and discussion
3.1 Relative density
The sintering density of ceramics is one of the important factors which effect the microwave dielectric properties; therefore, the bulk density and relative density of Zn(Ti1−xZrx)Ta2O8 ceramics are evaluated and presented in Fig. 1. For each composition (Fig. 1a), the relative densities first increased and then decreased after reaching a maximum value along with the temperature enhancement. The bulk density of ceramics samples raised gradually from 6.92 to 7.43 g/cm3 by increasing Zr4+, which was attributed to the higher density of ZnZrTa2O8 (7.59 g/cm3) than ZnTiTa2O8 (6.92 g/cm3). Additionally, the optimum sintering temperature of samples where the maximum densities were acquired could be summarized. The measured relative density (Fig. 2b), ranged from 96.01 to 97.99%, which indicated that all samples sintered at their optimum temperature were well-sintered. Further, with the substitution of Zr4+ (x = 0.2), the relative density of samples sharply enhanced to 97.99%, which demonstrated that a small amount Zr4+ could improve the sinterability of ceramics.
3.2 Phase and structure
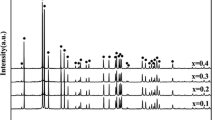
The XRD patterns of Zn(Ti1−xZrx)Ta2O8 ceramics sintered at their optimum temperature are shown in Fig. 2. Through indexing the patterns, the ceramics adopted the tetragonal cell of the triutile-type structure (JCPDS: #32–0702, P42/m n m (#136)) when x = 0. However, when x = 0.4–0.6, all patterns were matched perfectly with the orthorhombic cell of columbite-type structure (JCPDS: #48-0326, Pbcn (#60)). Finally, the monoclinic wolframite-type structure (JCPDS: #48-0324, P2/c (#13)) could be observed in the compositions of x = 0.8–1. It is worth noting that the ceramics sample formed a mixture of tri-rutile and columbite structure with the nominal composition of ZnTi0.8Zr0.2Ta2O8 rather than a single phase as x = 0.2. As shown in the insets of Fig. 2, the (110) peak of tri-rutile structure disappeared gradually, the (111) and (110) peak position of columbite structure shifted to lower degree and some new diffraction peaks of monoclinic wolframite structure emerged during the process of structure evolution. This structural evolution in the Zn(Ti1−xZrx)Ta2O8 ceramics system implied the nonnegligible change of structure parameters as well as their properties.
The lattice parameters and cell volumes of Zn(Ti1−xZrx)Ta2O8 ceramics are depicted in Fig. 3. From the results, it was found that there was a conspicuous variation on the cell volumes related to lattice parameters with Zr-substitution. Although a and b increased constantly with the increase of Zr4+ content, the c parameter, contributing most to the cell volume change in the compositions of x = 0–1, was responsible for the shrinkage of cell volume. Afterwards, the lattice parameters (both a, b and c) showed an upward trend. Therefore, the variation trend of cell volumes was similar to that of c. In addition, Fig. 4 shows the structural schematic diagrams of ZnTiTa2O8, ZnZrTa2O8 and Zn(Ti1−xZrx)Ta2O8 ceramics (0.4 ≤ x ≤ 0.6). For tri-rutile structured ZnTiTa2O8 ceramics (Fig. 4a), Zn2+, Ti4+ and Ta5+ were all divided into two types of cations (Zn1, Zn2, Ti1, Ti2, Ta1 and Ta2), respectively. The 2e Wyckoff position was distributed by Zn1(0.4)/Ti1(0.4)/Ta1(0.2) cations while Zn2(0.175)/Ti2(0.175)/Ta2(0.650) cations occupied the 4e Wyckoff position, whereas oxygen anions were located in 4f and 8j Wyckoff positions [5]. For monoclinic wolframite structured ZnZrTa2O8 ceramics (Fig. 4b), the 2e Wyckoff position was distributed by Zn/Zr cations, Ta cations occupied the 2f Wyckoff position and oxygen anions were located in 4 g Wyckoff position [1]. As for columbite structure (Fig. 4c), Zn/Zr/Ti cations occupied the 4c Wyckoff position, while Ta cations and O anions occupied the 8d Wyckoff position. Obviously, the number of oxygen octahedrons of ZnTiTa2O8 was almost twice as that of ZnZrTa2O8 or Zn(Ti1−xZrx)Ta2O8 (0.4 ≤ x ≤ 0.6) along the direction of c-axis. In other words, the lattice parameter along c-axis was absolutely distinct between them. This result was corroborated in Fig. 3. In short, the structural evolution in the Zn(Ti1−xZrx)Ta2O8 ceramics would have a strong influence on microwave dielectric properties.
3.3 Raman spectroscopy
In order to characterize the phase composition of Zn(Ti1−xZrx)Ta2O8 ceramics, Raman spectroscopy which was sensitive to the variation of structure was employed. Figure 5 displays the Raman spectra of Zn(Ti1−xZrx)Ta2O8 ceramics over the frequency range 50–1000 cm− 1. Three types of Raman spectra could be clearly observed, which demonstrated that there were three different structures existing in the Zn(Ti1−xZrx)Ta2O8 ceramics system, which was in coincidence with the XRD results. In fact, only few Raman peaks were depicted in Fig. 5 because some weak vibration modes could be overlapped or broadened [6]. Tri-rutile structured ZnTiTa2O8 ceramics had four strongest Raman peaks, including B1g mode (72 cm−1) caused by the rotating of [TaO6] oxygen octahedrons, Eg mode (386 cm−1) originated from the vibration of cations and rotation of oxygen octahedrons, Eg mode (457 cm−1) ascribed to the stretching vibration of Ti-O bond and A1g mode (697 cm−1) resulted from the stretching of Ta–O bond [13]. These modes were only detected from the pure phase of ZnTiTa2O8 ceramics. However, with a small amount of Zr-substitution (x = 0.2), it displayed quite difference type of vibration modes belonging to columbite structure, indicating that the main structure of the samples had changed at this composition. When transition took place from tri-rutile to columbite, the Eg mode (386 cm− 1) and A1g mode (697 cm− 1) belonging to the tri-rutile structure disappeared and the main peaks of columbite structure B2g (110 cm−1), B1g (171 cm−1), Ag (293 cm−1) emerged [14]. These modes were observed plainly from x = 0.2 to x = 0.6. Starting with x = 0.8, four basic modes appeared which were summarized of Bg mode (122 cm− 1) caused by lattice vibration, Bg mode (275 cm− 1) resulted from the stretching vibration of Zn–O bond, Ag mode (644 cm− 1) caused by Ta–O stretching vibration. Meanwhile, the mode at 858 cm− 1 originated from Zr–O symmetric vibration also was a prominent peak [6, 15]. Beyond that, it could also be observed that the Raman spectra peak of monoclinic wolframite structure gradually intensified along with increase of Zr4+. The variation of Raman spectra was consistent with XRD analysis shown in Fig. 2. It demonstrated that the overall structural evolution process was from tri-rutile to coexistence of tri-rutile and columbite, then to columbite and finally to monoclinic wolframite structure.
3.4 Morphological
The SEM images of Zn(Ti1−xZrx)Ta2O8 ceramics sintered at different temperatures are given in Fig. 6. The EDS analysis of points marked in Fig. 6 are listed in the Table 1. The ceramics exhibited a dense microstructure with grain size of 2–15 µm. For x = 0 (Fig. 6a), pure phase of ZnTiTa2O8 ceramic depicted a uniform distribution of 13–15 µm grain size. Whereas for x = 0.2 (Fig. 6b), evidently, the grain with different morphologies could be observed, and marked as B and C, respectively. EDS results illustrated the specimen formed a mixture phase of ZnTiTa2O8 and ZnTi0.8Zr0.2Ta2O8, which was consistent with XRD analysis. At 0.4 ≤ x ≤ 0.8, all the grains of the ceramic samples presented a polygonal morphology with grain size of 5–10 µm. As x increased to 1, the grains were compactly accumulated and the grain size (2–5 µm) decreased obviously. It means that the average grain size of pure phase of ZnZrTa2O8 ceramic was smaller than that of ZnTiTa2O8 ceramics.
3.5 Microwave dielectric properties
Dielectric constant of Zn(Ti1−xZrx)Ta2O8 ceramics are provided in Fig. 7. The dielectric constants of all compositions enhanced with the temperature increasing and then declined after almost reaching the maximum value at their optimum densification temperature, which shared a similar trend with densities. It was easy to accept that relative density had a strong effect on the dielectric constants [16, 17]. For samples with different composition (Fig. 7b), it could be evidently observed that the dielectric constant declined sharply in pace with the increase of Zr4+ content. For samples with Zr4+ free, the dielectric constant showed the maximum value of 43.35 which followed similar value as Park et al. reported [3]. For a minor Zr-substitution (x = 0.2), the dielectric constant dropped precipitously to 33.12, which was attributed to the structural transition of ceramics. In this composition, although the tri-rutile and columbite structures coexisted, the latter had assumed a dominant role. Generally, most columbite structured oxides possessed lower dielectric constant (17–25) [18,19,20]; thus, the dielectric constant decreased with the columbite structured ceramics formation. Upon further increasing Zr4+, the dielectric constant continued to decline. When Zr4+ completely replaced Ti4+(x = 1), monoclinic wolframite structured ZnZrTa2O8 was formed and showed a lower dielectric constant of 24.53. In this work, the dielectric constant of Zn(Ti1−xZrx)Ta2O8 ceramics declined gradually with increasing Zr4+ content. This phenomenon was contrary to the ionic dielectric polarizability analysis which dielectric constant should increase with the enhancement of Zr4+ content due to the higher ionic dielectric polarizability of Zr4+ (3.25 Å) than Ti4+ (2.93 Å) [6]. This deviation was mainly ascribed the dominant and decisive influence of structural evolution on the properties of ceramics.
a Dielectric constant of Zn(Ti1−xZrx)Ta2O8 ceramics as a function of sintering temperature; b the variety of dielectric constant of Zn(Ti1−xZrx)Ta2O8 ceramics versus Zr-substitution (x = 0, at 1275 °C; x = 0.2, at 1300 °C; x = 0.4, at 1325 °C; x = 0.6, at 1350 °C; x = 0.8, at 1300 °C; x = 0, at 1300 °C)
Figure 8 displays the Q × f values of Zn(Ti1−xZrx)Ta2O8 ceramics. For the samples with same composition, when x ≤ 0.2 (Fig. 8a), the variation trend of Q × f values was similar with that of density. However, when x ≥ 0.4 (Fig. 8b), Q × f values kept increasing as the rise of temperature, which mainly caused by the abnormal growth of grains at higher temperature. The total number of grain boundaries in ceramics decreased with grain size increasing. The defects caused by grain boundaries, such as pores, vacancies, and impurities also declined, thus reducing the extrinsic dielectric loss and showing higher Q × f values [21, 22]. For the samples with different Zr-substitution, it is obvious from Fig. 8b that Q × f values changed dramatically at two component points, which was x = 0.2 and x = 0.8, respectively. The main reason for this phenomenon was that there had been marked structural transitions at these two components. The maximum Q × f values of 44,048 GHz could be achieved at the compositions of x = 0.2.
Figure 9 exhibits the τf values of Zn(Ti1−xZrx)Ta2O8 ceramics. It was well recognized that the temperature coefficients of the resonance frequency of Zn(Ti1−xZrx)Ta2O8 ceramics declined gradually, and could gain the closest value to zero at the compositions of x = 0.2. For samples with Zr4+ free, the τf values reached 77.1 ppm/°C, and for a minor Zr-substitution (x = 0.2), the τf values sharply declined to − 11.9 ppm/°C. Finally, when Zr4+ completely replaced Ti4+(x = 1), the τf value of samples was − 35.22 ppm/°C. Generally, τf value was related to the microstructure, composition, and structure of the materials [23]. In order to reveal the relationship between τf values and the structure of ceramics, the microwave dielectric properties of ceramics with tri-rutile, columbite, and wolframite structures are summarized in Table 2. Referring to the published literatures, most tri-rutile structured ceramics had positive τf values (74–111.54 ppm/°C), while ceramics with columbite and wolframite structure exhibited negative τf values (− 38 to − 56.54, − 24.96 to − 32 ppm/°C, respectively). Herein, τf values of these three pure phase ceramics were basically consistent with the results in Table 2. As for samples with the coexistence of tri-rutile and columbite structure (x = 0.2), τf value was adjusted to − 11.9 ppm/°C due to the composite of two phases. The τf values of composites could be calculated by the following mixing rule [27, 28]:
where τf was the temperature coefficient of resonant frequency of composite ceramics. \({\tau}_{f_1}\) and \({\tau}_{f_2}\) were the temperature coefficient of resonant frequency of two different phases of ceramics. \({v}_{1}\) and \({v}_{2}\) were the volume fraction of the two phases, respectively. According to the XRD results, the volume fraction of columbite phase (~ 83.8%) in ceramics was larger than that of tri-rutile phase (~ 16.2%) when x = 0.2. Therefore, it could be estimated that the τf value should be a negative value closing to 0. All in all, in this work, the τf values were significantly affected by structural transition in Zn(Ti1−xZrx)Ta2O8 ceramics.
4 Conclusions
In this work, the sintering behavior, crystal structure, Raman spectroscopy, microstructure, and microwave dielectric properties of Zn(Ti1−xZrx)Ta2O8 (x = 0–1) ceramics were studied. It was found that there were three structure types (tri-rutile, columbite, and monoclinic wolframite) existing in the Zn(Ti1−xZrx)Ta2O8 ceramics system. For samples with Zr4+ free (x = 0, ZnTiTa2O8), they possessed the tri-rutile-type structure. When 0.4 ≤ x ≤ 0.6, a columbite-type structure was detected. For x ≥ 0.8, all samples remained monoclinic wolframite-type structure. However, a mixture of tri-rutile and columbite structure was observed when x = 0.2. The structural evolution process was from tri-rutile to coexistence of tri-rutile and columbite, then to columbite and finally to monoclinic wolframite structure with the increase of Zr4+ content. The variation of εr, improvement of Q × f values and betterment of τf values of Zn(Ti1−xZrx)Ta2O8 ceramics were influenced strongly by the structural transition. Typically, ZnTi0.8Zr0.2Ta2O8 (x = 0.2) ceramics exhibited excellent microwave dielectric properties of εr ~ 29.9, Q × f ~ 44,048 GHz, and τf ~ − 11.9 ppm/°C at Ts = 1300 °C.
References
X.S. Lyu, L.X. Li, S. Zhang, H. Sun, S. Li, J. Ye, B.W. Zhang, J.T. Li, J. Eur. Ceram. Soc. 36, 931–935 (2016)
M.J. Wu, Y.C. Zhang, M.Q. Xiang, J. Mater. Sci. Mater. Electron. 29, 14471–14478 (2018)
J.H. Park, Y.J. Choi, S. Nahm, J.G. Park, J. Alloys Compd. 509, 6908–6912 (2011)
N. Kumada, N. Koike, K. Nakanome, S. Yanagida, T. Takei, A. Miura, E. Magome, C. Moriyoshi, Y. Kuroiwa, J. Asian Ceram. Soc. 5, 284–289 (2017)
N. Kumada, N. Koike, K. Nakanome, S. Yanagida, T. Takei, A. Miura, E. Magome, C. Moriyoshi, Y. Kuroiwa, J. Asian Ceram. Soc. 6, 247–253 (2018)
Y. Zhang, Y.C. Zhang, M.Q. Xiang, J. Eur. Ceram. Soc. 36, 1945–1951 (2016)
C.H. Hsu, C.J. Huang, J. Alloys Compd. 587, 45–49 (2014)
M.J. Wu, J.D. Chen, Y.C. Zhang, Funct. Mater. Lett. 12, 1950020–1950023 (2019)
Q.W. Liao, L.X. Li, X. Ding, Solid State Sci. 14, 1385–1391 (2012)
B.W. Hakki, P.D. Coleman, IRE Trans. Microw. Theory 8, 402–410 (1960)
W.E. Courtney, IEEE Trans. Microw. Theory 18, 476–485 (1970)
Y. Kobayashiy, M. Katoh, IEEE Trans. Microw. Theory 33, 586–592 (1985)
H.Y. Yang, S.R. Zhang, Y.W. Chen, H.C. Yang, Y. Yuan, E.Z. Li, Inorg. Chem. 58, 968–976 (2019)
D.P. Xu, Y. Liu, Q. Zhou, T. Cui, H. Yuan, W. Wang, Z. Shi, L. Li, J. Alloys Compd. 618, 694–699 (2015)
H.T. Wu, Z.B. Feng, Q.J. Mei, J.D. Guo, J.X. Bi, J. Alloys Compd. 648, 368–373 (2015)
C.F. Tseng, J. Eur. Ceram. Soc. 34, 3641–3648 (2014)
C.F. Tseng, J. Eur. Ceram. Soc. 35, 383–387 (2015)
R.C. Pullar, J.D. Breeze, N.M. Alford, J. Am. Ceram. Soc. 88, 2466–2471 (2005)
Y.C. Liou, C.Y. Shiue, M.H. Weng, J. Eur. Ceram. Soc. 29, 1165–1171 (2009)
H.J. Lee, I.T. Kim, K.S. Hong, Jpn. J. Appl. Phys. 36, 1318–1320 (1997)
Y. Zhang, Y.C. Zhang, J. Alloys Compd. 683, 86–91 (2016)
N. Ichinose, T. Shimada, J. Eur. Ceram. Soc. 26, 1755–1759 (2006)
Y.C. Chen, Y.W. Zeng, J. Alloys Compd. 481, 369–372 (2009)
S.Y. Wang, J.D. Chen, Y.J. Zhang, Y.C. Zhang, J. Alloys Compd. 805, 852–858 (2019)
M.J. Wu, Y.C. Zhang, J.D. Chen, M.Q. Xiang, J. Alloys Compd. 747, 394–400 (2018)
S.D. Ramarao, V.R.K. Murthy, Scr. Mater. 69, 274–277 (2013)
C.L. Huang, M.H. Weng, Mater. Res. Bull. 36, 2741–2750 (2001)
Y. Lv, R.Z. Zuo, Y. Cheng, C. Zhang, J. Am. Ceram. Soc. 96, 3862–3867 (2013)
Acknowledgements
This work has been financially supported by the National Natural Science Foundation of China (No. 51772022) and Fundamental Research Funds for the Central Universities (FRF-GF-18-005A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Xiao, B., Li, J. et al. Structural evolution, Raman spectra, and microwave dielectric properties of Zr-substituted ZnTiTa2O8 ceramics. J Mater Sci: Mater Electron 31, 10298–10305 (2020). https://doi.org/10.1007/s10854-020-03577-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03577-3