Abstract
Rare earth-doped Ba0.85Ca0.15Zr0.1Ti0.9O3 (BCZT) ceramics demonstrated moderately improved properties rather than undoped counterpart. In this investigation, we report the synthesis and characterization of La3+-doped BCZT (x = 0.00, 0.02, 0.04, 0.06, and 0.08) single-phase crystals prepared by high-temperature (~ 1300 °C) solid-state reaction technique. Rare earth ions addition to BCZT affects its microstructure, phase transition, and electrical properties. All compounds were crystallized in tetragonal structure at room temperature. Scanning electron microscopy (SEM) recorded the microstructural analysis of the compounds. Temperature-dependent dielectric and impedance properties of the sintered pellet were estimated at chosen frequencies as a function of temperature. Here, efforts were made to improve the electrical properties of the material with the addition of La3+ in the BCZT system. The obtained data revealed that Curie temperature (TC) diminishes with the introduction of La3+ in BCZT ceramics. Besides, the diffusivity analysis and conductivity studies were used for better comprehension of the electrical processes in the materials. The obtained lead-free ceramics with enhanced electrical properties could be utilized in the fabrication of electronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ceramics which are based on lead (e.g. lead zirconium titanate, PbTiO3–PbZrO3 (PZT)) have influenced the field of ferroelectrics globally. But, as lead oxides are lethal, it induces grave environmental issues. Consequently, perovskite oxides which are lead-free have been extensively explored on account of their exceptional dielectric, piezoelectric, ferroelectric, and electrical properties [1,2,3]. These perovskites are positioned in an unique group of industrially chief materials appropriate for their applications in diverse fields [4]. The structural, microstructural, and electrical properties of these materials have been broadly investigated as they facilitate in accessing a variety of vital parameters concerned with the manufacture of chief components in electronic devices. A sequence of lead-free ceramics has been studied in the search for novel dielectrics [5,6,7,8]. It is well recognized that ceramic processing and doping are chief factors that control their structure, composition, and the electrical properties [9,10,11], which provides the outlook to modify the electrical properties further. In the field of BaTiO3 (BT)-based dielectric ceramic materials, La3+ ions are recognized to solely substitute at Ba sites [12,13,14]. The dielectric properties of BT ceramics have been found to be improved by doping with La3+ and several literatures available also suggest the improved properties of BT on doping with La3+ [15, 16]. It has also been reported that the La-doped BT ceramics have superior dielectric constant and its transition temperature (TC) decreased as the La content increases [17] which is attributed to the fact that La acts as a donor because it occupies the Ba site. Also, with an oxidation state of 3 + and less ionic radius (1.36 Å) compared to that of Ba2+ (1.61 Å), it will only occupy A site in the perovskite lattice. La3+ is more soluble into the BT lattice in comparison to other rare earths which are consequential in variation of symmetry and grain size [13]. Hence, it is apparent that La3+-doped BT is an ideal candidate which can be utilized as a dielectric in capacitors [18,19,20,21]. In the recent years, BZT ceramic engrossed extraordinary importance owing to its excellent properties which is favorable for device applications [22,23,24]. Lately, there have been reports of improved dielectric properties of BZT ceramics with doping of rare earth ions [25,26,27,28]. In particular, it has been studied that with the increase in the lanthanide content, the diffusivity increases and the electrical properties are enhanced in the BZT system [17, 29,30,31]. Later, in 2009, excellent dielectric property in Barium calcium zirconate titanate (BCZT) ceramics was reported which shifted the attention from BZT of the researchers worldwide [32]. The effect of rare earth oxides on various properties of BCZT ceramics was broadly studied in the past few years [33, 34]. However, there is not much research on La3+-doped BCZT ceramics. Therefore, in the present work, the effect of La3+ content on the TC, microstructure, and electrical properties of Ba0.85Ca0.15Ti0.9Zr0.1O3 − xLa (x = 0.00, 0.02, 0.04, 0.06 and 0.08) ceramics synthesized by solid-state reaction technique is reported. Investigation of these properties as a function of La3+ content is carried out for potential applications.
2 Experimental
(Ba0.85Ca0.15)1−xLa2x/3(Zr0.1Ti0.9O3) polycrystalline samples with x = 0.00, 0.02, 0.04, 0.06, and 0.08 (BCZT-xLa) were synthesized by solid-state reaction technique by means of pure carbonates and oxides. BaCO3 (CDH, 98%), CaCO3 (CDH, 98%), TiO2 (CDH, 99%), ZrO2 (CDH, 99%), and La2O3 (CDH, 99%) were used as precursors in preferred stoichiometric proportions. All the ingredients were blended meticulously in air and wet (i.e., acetone) atmosphere for 2 h each by utilizing an agate mortar and afterward it was evaporated by gradual evaporation. The air-dried mixture was calcined at 1250 °C for 4 h. After repeating the procedure of calcination and grinding, homogeneous fine particles of the compound were attained. The equation for the reaction is given by:
X-ray diffraction (XRD) technique is executed to confirm the compound formation by utilizing Ultima IV Model—Rigaku, Japan, worked at room temperature in a wide Bragg angles range 2θ where 20 ≤ 2θ ≤ 80°. The powdered samples after calcination were accordingly cold pressed at 5 × 106 N/m2 pressure into cylindrical pellets of under 1 mm thickness and 10 mm diameter by utilizing a hydraulic press. For diminishing the brittleness in the pellets, polyvinyl alcohol (PVA) which behaves as a binder was included. Sintering of the pellets was performed at an advanced temperature 1300˚C for 4 h in air atmosphere. Both faces of the sintered pellets were cleaned utilizing fine emery paper to ensure they are parallel and flat. Scanning electron microscope (HITACHI SU3500) was used to record the microstructures of the unpolished pellets. The flat and parallel face of the pellet was layered by silver paste having high purity for the electrical characterizations of the materials. After electroding the pellet, it was dried for 1 h at 200 °C to clear away the moisture, if present. Prior to taking any estimation, the pellet was cooled at room temperature. HIOKI 3532 LCR Hitester, a computer-controlled impedance analyzer, was executed for examining dielectric properties of the samples in the frequency range from 100 Hz to 1 MHz.
3 Results and discussion
3.1 Structural study
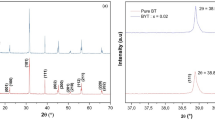
The X-ray diffraction pattern of BCZT-xLa at room temperature is shown in Fig. 1. No remarkable change is noticed in the peak position with the substitution of La in BCZT. Sharp and distinct peaks noticed in the X-ray diffraction pattern confirm that a good crystalline material is formed. The monophasic nature of the samples is noticed from the XRD patterns as there are no secondary phases or impurity peaks corresponding to the reactant oxides. There is a small difference in the peak intensity which might perhaps result as a consequence of particle size variation or La3+ substitution at the Ba site. From the XRD pattern of the samples, indexing of the prominent reflection peaks is done and subsequently by means of “POWD” (standard computer program), the lattice parameters in diverse crystal systems and pattern were found out [35]. A suitable unit cell in the tetragonal crystal system was selected on the finest accord between the observed (dobs) and calculated (dcal) inter planar spacing d where \(\Sigma \Delta d = d_{{{\text{obs}}}} - d_{{{\text{cal}}}} =\) minimum. By using “POWD”, the refinement of lattice parameters of the selected unit cell was carried out. The lattice parameters (in Å), c/a ratio, and unit cell volume V (in Å3) for different La concentrations (x) are represented in Table 1. As the ionic radii of La3+(1.36 Å) is less than that of Ba2+(1.61 Å), it is expected that there should not be any considerable change in lattice parameters as well as unit cell volume. From Table 1, it has been observed that, the variation of the unit cell volume is between 63 and 64 (Å3), i.e., within 1 Å3. Hence, there is not any appreciable change in the lattice parameters and unit cell volume with La-substitutions [36, 37]. This variation is well within the experimental errors. On the other hand, c/a ratio (which is related to the tetragonality) decreases with an increase in La concentrations and the tetragonality is related to the phase transition temperature [38]. Hence, the decrease in the phase transition temperature is well correlated with the decrease in the tetragonality [39].
By using Scherrer’s equation and full width at half maxima of the peak, the average crystallite size (P) of samples was estimated. The average crystallite sizes (P) of all the samples are tabulated in Table 1. The diameter of all the samples lies in the range 18–29 nm. The average crystallite size does not vary considerably with substituent La concentration [40, 41]. The decrease in TC (as shown in Fig. 3) also indirectly confirms the existence of La in the lattice. It is expected that the reduced lattice constant of the perovskite unit cell could possibly result from the incorporation of La3+ which is smaller as compared to the larger Ba2+. There was no considerable variation in the lattice parameters of the samples with the change in concentration of La3+ [42, 43].
4 Morphological study
SEM images of the sintered BCZT-xLa ceramics (x = 0.00, 0.02, 0.04, 0.06 and 0.08) are shown in Fig. 2. We notice that densely packed grains of diverse shapes and sizes having less porosity are spread above the whole surface of the samples which confirms the polycrystallinity of the samples. From the micrographs, it is evident that these grains are alienated from one another by means of grain boundaries. For the estimation of the average grain size of the samples, linear intercept method was implemented, where a random straight line was drawn through the micrograph and the number of grain boundaries intersecting the line was counted. The average grain size was found by the help of the following formulation [44, 45]:
where the actual line length = measured length/magnification. The average grain size was calculated which is approximately 2–3 µm. As the dopant concentration is increased, the grain size becomes more homogeneous. Lower diffusivity may possibly arise because of the rare earth ions which are identified to suppress the growth of grains in perovskites [28]. The decrease in the aspect ratio is an additional distinguished characteristic in La-doped samples. As the La content increases, there is a slight reduction in the ‘thickness’ of plate-like grains whereas a considerable reduction in the ‘width’ is noticed. As these randomly oriented grains having a high aspect ratio intrude on each other, the process of densification is hindered. This results in higher porosity mainly at the grain boundaries. The La-doped samples show a small improvement in the density which is attributed to the decrease in the aspect ratio of the grains. The addition of La3+ in Ba2+ causes A site vacancy, and during the process of sintering this favors the diffusion of constituent ions [42].
5 Dielectric studies
Figure 3 demonstrates the temperature (room temperature—200 °C) dependence of relative dielectric constant (εr) at 1 kHz, 10 kHz, 50 kHz, and 100 kHz for BCZT-xLa. The ferroelectric-paraelectric phase transition (TC) is noticed in some materials. The TC value for compositions of La is considerably less than the undoped BCZT [46, 47]. The value of TC decreases which may possibly be due to the La3+ ion occupancy as a donor impurity on Ba2+/Ca2+ sites [48]. The doping of La3+ results in a decrease of TC along with significant increase in dielectric constant [36]. A reduction of the tetragonal unit cell is caused when Ba2+ is substituted by lanthanide ion (Ln3+), and this result increases with increasing of the ionic radii of Ln3+. This reduction leads to a small shift of the tetravalent cation, Ti4+ or Zr4+, outside the center of the oxygen octahedron, and a corresponding decrease of maximum temperature. This can also be explained as a deformation in the crystalline lattice which may be formed as a result of substitution of Ln3+ ions. This substitution is established on A site vacancy defect compensation model. This model suggests that only single cationic vacancy is produced from two lanthanide ions placed on the A site which sustains the charge neutrality in perovskites. In Ln-doped BZT ceramics, there is a rise in the short range harmonic restoring force which is caused by the distortion of the structure of lattice. As the concentration of Ln3+ ions increases, the amount of cationic vacancies also increases, and as a result, the value of TC decreases [31]. For all composition of x, it is noticed that TC is independent of frequency signifying the non-relaxor characteristic of the synthesized materials. The value of TC for BCZT-xLa for the composition x = 0.00, 0.02, and 0.04 was found to be 95 °C, 80 °C, and 30 °C whereas with the increase in concentration of La (specifically for x = 0.06 and 0.08), the TC value further decreases and is below the room temperature which can be noticed from the figure. The value of εr increases significantly with the addition of La [37]. It has also been reported that in denser ceramics and large grains, the domain walls are relatively free and the dielectric constant (εr) increases for such samples [49]. As the La content increases, there is an evident increase in the dielectric constant which may possibly be due to the fact that the temperature of dielectric anomaly (Tc) approaches the room temperature. The beginning of this dielectric anomaly could contribute in the dielectric constant enhancement [42].
Figure 4 illustrates the dielectric loss (ε″) variation as a function of temperature at 100 kHz for BCZT-xLa. Here, we notice that even after the addition of La in the BCZT lattice, the dielectric loss value remains small. The movement of oxygen vacancies is restrained by the La3+ dopants which contribute in the decrease of dielectric loss value [42].
6 Diffusivity analysis
In order to comprehend the dielectric nature in ceramics in an enhanced approach, diffusivity analysis is implemented. Modified Curie–Weiss law was given with the purpose of explaining the ferroelectric phase transition diffuseness, and the derivation of ‘εr’ can be obtained with the help of following equation:
where \(\varepsilon_{{\text{r}}}\) is the relative permittivity and \(\varepsilon_{{{\text{r}}\max }}\) is the maximum \(\varepsilon_{r}\) while \(T_{{\text{m}}}\) corresponds to temperature at \(\varepsilon_{{{\text{r}}\max }}\). The value γ = 1 corresponds to an ideal Curie–Weiss behavior, while γ = 2 corresponds to the ideal relaxor ferroelectric, whereas γ value between 1 and 2 indicates that the phase transition is diffused [50]. Figure 5 illustrates the plot between log(\(1/\varepsilon_{{\text{r}}} - 1/\varepsilon_{{{\text{r}}\max }}\)) and log \(\left( {T - T_{{\text{m}}} } \right)\) for BCZT-xLa (x = 0.00, 0.02, and 0.04) ceramics at 100 kHz. The diffuseness value obtained from the slope of the fitted curve changes a little with the concentration of La. At higher temperatures (\(T > T_{{\text{m}}}\)), the modified Curie–Weiss law is useful. However, at higher temperatures, the dielectric properties are affected significantly by conductivity owing to mobile oxygen vacancies. The γ value obtained for different La concentrations shows that the material exhibits diffused ferroelectric behavior. The structural disorder and compositional fluctuation is generally accountable for the broadness or diffuseness [51]. We also notice a decline in γ value with the increasing concentration of La in BCZT which could be the result of decrease in the number of oxygen vacancies and consequently, the deviation from linear behavior is minimized. The γ values for different concentrations of La are given in Table 1.
7 Complex impedance spectroscopy
An easy and non-destructive method called complex impedance spectroscopy (CIS) [52] is utilized in order to understand the electrical processes including the conduction phenomenon in the materials. It is used to study impedance and other related parameters. Additionally, it is a valuable approach for scrutinizing the effect of grain, grain boundary, and electrode polarization on various parameters. Complex impedance and different parameters associated with impedance are evaluated by using different relations [53, 54].
Figure 6 shows the frequency dependent real part of impedance (Z′) for BCZT-xLa at 425 °C and 450 °C. For all concentrations of La, the value of Z′ decreases monotonously as the frequency is increased. At low temperature, the impedance value is high which gradually decreases as the frequency is increased in the lower frequency area. From the figure, it is evident that the samples exhibit the negative temperature coefficient of resistance (NTCR) behavior as there is a decreasing tendency in Z′ with the rise in temperature in the lower-frequency region. But the low-temperature data merges in the higher-frequency area perhaps attributable to the released space-charge polarization as an effect of the decreasing barrier property in the ceramics. At the high-frequency region, the direction of flow is prevented inside the dielectric medium, accumulation of charges occurs at the grain boundaries and as a result, there is an overall decrease in the impedance value at higher frequencies [55]. The decreasing rate of Z′ with the enhancement in frequency and temperature portrays the probability of increase in conductivity. Hence, there will be an increase in the electrical conduction as the temperature is increased [56].
The variation of the imaginary part of the impedance Z″ with frequency at different temperatures (425 °C and 450 °C) for BCZT-xLa is illustrated in Fig. 7. The fundamental characteristics of the Z″ plot are the occurrence of peak at a distinct frequency (relaxation frequency), decrease in Z″ value toward the high-frequency region with relaxation frequency shift, and the broadening nature of peak is apparent when temperature is raised. The peak broadening as the temperature rises indicates the existence of electrical relaxation phenomenon in the material that is dependent on temperature. The asymmetric peak broadening confirms the existence of electrical processes in addition to the spreading of relaxation time. The substitution of La affects the materials’ electrical processes which are evident in terms of peak shifting toward lower frequency, peak broadening and asymmetry increases, and the increase in Z″ value. In the high-frequency region, a sole relaxation peak is noticed which is associated with the grain/bulk effect. With addition of dopant in the system, the relaxation process becomes more prominent. The upsurge in peak broadening and asymmetry on La addition indicates a deviation from the ideal Debye-type behavior in addition to an increase of relaxation time distribution. At low temperature, the relaxation is perhaps caused by the immobile charge carriers, whereas at higher temperature, vacancies/defects in the material are responsible [57].
The complex impedance plots (Nyquist plots) of BCZT-xLa are illustrated in Fig. 8. The figure shows single semi-circular arcs for the temperature range (400–500 °C) which implies that the polarization mechanism takes place owing to presence of grains. When the temperature rises, there is a reduction in radii of the curvatures in the materials which indicates weak insulating property. Moreover, we note a decline in grain/bulk resistance (Rb) value on escalating the temperature [58]. Information regarding the types of electrical processes going on in the material is given by the intercept on the x axis and the relationship among the arcs. The occurrence of intra-grains is possibly liable for the electrical processes at elevated temperature [59]. The fitting of Nyquist plots is performed by using ZSIMP WIN version 3.21 [60, 61]. Table 2 gives the calculated bulk resistance (Rb) and bulk capacitance (Cb) from the fitted curves. The NTCR characteristic is noticeable in the material from the decrement in value of Rb with the temperature rise.
Figure 9 illustrates the variation of relaxation time as a function of reciprocal of absolute temperature. The relaxation time (τb) is estimated by means of Z″ and M″ spectroscopy. The plots follow Arrhenius behavior: τb = τ0 exp (−Ea/KBT) where Ea is the activation energy, τ0 is the pre-exponential factor, KB is Boltzmann constant, and T is the absolute temperature. The rate of b exponentially reduces as the temperature increases and meets the Arrhenius relationship for all samples. From Z″ spectra, the calculated Ea values correspond to the localized conduction which is the dielectric relaxation whereas the Ea values found out from M″ spectra is the non-localized conduction which is the long-range conduction [62]. The activation energies calculated from Z″ spectra and M″ spectra are given in Table 3. About equal Ea values estimated using Z″ and M″ spectra shows that the mechanism that causes the conductivity process in all the samples are similar and identical charge carriers are liable for conductions (localized and non-localized) [63].
8 DC conductivity
The variation of dc conductivity as a function of inverse of absolute temperature is shown in Fig. 10. From the Nyquist plots, the bulk conductivity value is estimated by employing the equation: σdc = t/RbA, where ‘A’ and ‘t’ correspond to the surface area and thickness and ‘Rb’ stands for the grain resistance of the material. With an increase in temperature, dc conductivity (σdc) value increases which confirm the NTCR aspect of the material. Moreover, the dc conductivity plots follow Arrhenius relation [54]: σdc = σ0exp (− Ea/KBT). The slope of the plot gives hopping electron activation energy (Ea) values for different compositions of La as shown in Table 3 which is almost similar to Ea values estimated from relaxation time plots (as shown in Fig. 9) [64]. Hence, it is apparent that almost similar carriers account for the different processes (relaxation and conduction).
9 AC conductivity
It is essential to consider the ac conductivity in order to understand the electrical properties of the material. To find out the ac conductivity of the samples, the following relation is used: \(\sigma_{{{\text{ac}}}} = \omega \varepsilon_{{\text{r}}} \varepsilon_{0} \tan \delta\). Figure 11 illustrates ac conductivity variation with frequency at 425 °C and 450˚C for BCZT-xLa. By using Jonscher’s power law [59], the conductivity \(\sigma \left( \omega \right)\) can be estimated: \(\sigma \left( \omega \right) = \sigma_{{{\text{dc}}}} + A\omega^{n}\) [54]. Here, ‘A’ and ‘n’ are temperature-dependent factors. Three main dispersion regions are observed in case of ac conductivity plot. Firstly, the lower-frequency area where conductivity varies as a consequence of space-charge polarization. Secondly, the moderate frequency region contributing toward the dc conductivity (σdc) which arises due to the long-range translational movement of ions. Lastly, the region at high frequency having variable slopes [65]. There is steady conductivity in conjunction with a distinct frequency plateau on La doping in the lower-frequency region [66]. We notice that the conductivity rises as the temperature is enhanced. The conductivity also increases nonlinearly with frequency as seen in the figure (moderate region) and in the high-frequency region; it is followed by frequency dispersion [67]. Mobility of the charge carriers increases remarkably which is responsible for the enhancement of ac conductivity. Hopping frequency is identified as the frequency where the dispersion occurs [68] and we can note from the figure that the hopping frequency approaches the higher frequency domain as the temperature is increased. With the enhancement in frequency, the active charge carriers’ situated among the trapped locations exhibit hopping mechanism. The overall conductivity σ(ω) which depends on frequency is studied using the following relation [69]
Here, σdc (0) represents the (frequency independent) dc conductivity while σac (ω) represents the ac conductivity due to the hopping mechanism.
10 Conclusion
Single-phase polycrystalline BCZT-xLa ceramics were synthesized by high-temperature solid-state reaction technique. The phase transition (TC) decreases when the La3+ concentration is increased in the BCZT system by virtue of Ba2+ substitution with La3+ ion with the probable formation of cation vacancies. High dielectric constant (εr) with low dielectric loss is exhibited with reduction in doping for the BCZT-xLa compounds. The diffusivity calculations reveal that with addition of La3+ in the BCZT system, there is a decline in the number of oxygen vacancies. The Nyquist plots show a decrease in resistivity as the temperature increases. The estimated activation energy values from the relaxation graphs show that the charge carriers accountable for the relaxation and conduction processes are similar. The temperature-dependent dc conductivity also indicates the NTCR behavior in the materials and follows the Arrhenius equation. The frequency-dependent ac conductivity is governed by Jonscher’s universal power law.
References:s
S. Kumar, R. Kurchania, A. Kumar, Ceram. Int. 45, 17137–17143 (2019)
S. Ramesh, D. Ravinder, K.C.B. Naidu, N.S. Kumar, K. Srinivas, D.B. Basha, B.C. Sekhar, Biointerface Res. Appl. Chem. 9, 4205–4216 (2019)
L. Pardo, J. Ricote, Multifunctional Polycrystalline Ferroelectric Materials: Processing and Properties, vol. 806 (Springer, Berlin, 2011)
L. Jin, F. Li, S. Zhang, J. Am. Ceram. Soc. 97, 1–27 (2014)
K.C.B. Naidu, V.N. Reddy, T.S. Sarmash, D. Kothandan, T. Subbarao, N.S. Kumar, J. Aust. Ceram. Soc. 55, 201–218 (2019)
A. Zeb, S.J. Milne, J. Eur. Ceram. Soc. 34, 1727–1732 (2014)
I. Zouari, Z. Sassi, L. Seveyrat, N. Abdelmoula, L. Lebrun, H. Khemakhem, Ceram. Int. 44, 8018–8025 (2018)
A. Grünebohm, T. Nishimatsu, Phys. Rev. B 93, 134101 (2016)
D. Kothandan, R.J. Kumar, K.C.B. Naidu, J. Asian Ceram. Soc. 6, 1–6 (2018)
D. Kothandan, R.J. Kumar, M. Prakash, K.C.B. Naidu, Mater. Chem. Phys. 215, 310–315 (2018)
X. Liu, M. Zhu, Z. Chen, B. Fang, J. Ding, X. Zhao, H. Xu, H. Luo, J. Alloys Compd. 613, 219 (2014)
A. Zhang, Y.F. Qu, Trans. Nonferrous Met. Soc. China 22, 2742–2748 (2012)
M. Ganguly, S.K. Rout, T.P. Sinha, S.K. Sharma, H.Y. Park, C.W. Ahn, I.W. Kim, J. Alloys Compd. 579, 473–484 (2013)
S.K. Ghosh, M. Ganguly, S.K. Rout, T.P. Sinha, Eur. Phys. J. Plus. 130, 68 (2015)
D.Y. Lu, Y.Y. Peng, X.Y. Yu, X.Y. Sun, J. Alloys Compd. 681, 128–138 (2016)
M. Prakash, R.J. Kumar, K.C.B. Naidu, Mater. Res. Express 7, 015037 (2020)
S.B. Reddy, K.P. Rao, M.S.R. Rao, J. Alloys Compd. 481, 692–696 (2009)
Y. Wenhu, P. Yongping, C. Xiaolong, W. Jinfei, J. Phys. Conf. Ser. 152, 012040 (2009)
A. Honda, S. Higai, Y. Motoyoshi, N. Wada, H. Takagi, Jpn. J. Appl. Phys. 50, 09NE01 (2011)
Z. Xinle, M. Zhimei, X. Zuojiang, C. Guang, J. Rare Earth 24, 82 (2006)
Z. Valdez-Nava, S. Guillemet-Fritsch, Ch Tenailleau, T. Lebey, B. Durand, J.Y. Chane-Ching, J. Electroceram. 22, 238 (2009)
C. Ang, Z. Yu, Z. Jing, R. Guo, A.S. Bhalla, L.E. Cross, Appl. Phys. Lett. 80, 3424 (2002)
T. Maiti, R. Guo, A.S. Bhalla, Appl. Phys. Lett. 90, 182901 (2007)
Z. Yu, C. Ang, R. Guo, A.S. Bhalla, J. Appl. Phys. 92, 1489 (2002)
Y. Wang, L. Li, J. Qi, Z. Gui, Ceram. Int. 28, 657 (2002)
D. Shan, Y.F. Qu, J.J. Song, Solid State Commun. 141, 65 (2007)
S.B. Reddy, M.S.R. Rao, Appl. Phys. Lett. 91, 022917 (2007)
X. Chou, J. Zhai, H. Jiang, X. Yao, J. Appl. Phys. 102, 084106 (2007)
C. Ostos, L. Mestres, M.L. Martinez-Sarrion, J.E. Garcia, A. Albareda, R. Perez, Solid State Sci. 11, 1016 (2009)
X. Diez-Betriu, J.E. Garcia, C. Ostosc, A.U. Boya, D.A. Ochoa, L. Mestres, R. Perez, Mater. Chem. Phys. 125, 493–499 (2011)
S. Mahajan, D. Haridas, K. Sreenivas, O.P. Thakur, C. Prakash, Mater. Lett. 97, 40–43 (2013)
W. Liu, X. Ren, Phys. Rev. Lett. 103, 257602 (2009)
P. Parjansri, U. Intatha, S. Eitssayeam, Mater. Res. Bull. 65, 61 (2015)
J. Gao, Q. Li, H. Liu, J. Shim, Q. Yan, Y. Zhang, X. Chu, Ceram. Int. 41, 2497 (2015)
E.W. Powd, An interactive Powder diffraction data interpretation and indexing Program, Ver 2.1, School of Physical Science, Finders University of South Australia, Bedford Park, S.A. 5042, Australia.
Q. Zhang, X. Zhu, Y. Xu, H. Gao, Y. Xiao, D. Liang, J. Zhu, J. Zhu, D. Xiao, J. Alloys Compd. 546, 57–62 (2013)
M.M.V. Petrović, J.D. Bobić, T. Ramoška, J. Banys, B.D. Stojanović, Mater. Charact. 62, 1000–1006 (2011)
S.M. Bobade, D. Gulwade, A.R. Kulkarni, P. Gopalan, J. Appl. Phys. 97, 074105 (2005)
R. Köferstein, L. Jaeger, M. Zenkner, S.G. Ebbinghaus, Mater. Chem. Phys. 119, 118–122 (2010)
A. Manohar, C. Krishnamoorthi, J. Crystal Growth 473, 66–74 (2017)
A. Manohar, C. Krishnamoorthi, K.C.B. Naidu, C. Pavithra, Appl. Phys. A 125, 477 (2019)
S. Kumar, K.B.R. Varma, J. Phys. D 42, 075405 (2009)
I. Ramovatar, S. Coondoo, N. Satapathy, N.P. Kumar, J. Electron. Mater. 47, 5870–5878 (2018)
K.C.B. Naidu, S.R. Kiran, W. Madhuri, Mater. Res. Bull. 89, 125–138 (2017)
N.S. Kumar, R.P. Suvarna, K.C.B. Naidu, Ceram. Int. 44, 18189–18199 (2018)
J. Hao, W. Bai, W. Li, J. Zhai, J. Am. Ceram. Soc. 95, 1998–2006 (2012)
X. Lu, B. Fang, S. Zhang, N. Yuan, J. Ding, X. Zhao, F. Wang, Y. Tang, W. Shi, H. Xu, H. Luo, Func. Mater. Lett. 10, 1750046 (2017)
M.T. Buscaglia, V. Buscaglia, M. Viviani, P. Nanni, M. Hanuskova, J. Eur. Ceram. Soc. 20, 1997–2007 (2000)
K. Okazaki, K. Nagata, J. Am. Ceram. Soc. 56, 82–86 (1973)
P. Khushbu, V.K. Kumar, J. Alloys Compd. 731, 760–765 (2018)
G.N. Bhargavi, A. Khare, T. Badapanda, M.S. Anwar, N. Brahme, J. Mater. Sci Mater. Electron. 29, 11439–11448 (2018)
J.R. Macdonald, Impedance Spectroscopy (Wiley, Emphasizing Solid Materials and Systems. New York, 1987)
B.N. Parida, P.R. Das, R. Padhee, R.N.P. Choudhary, J. Phys. Chem. Solids. 73, 713–719 (2012)
S. Saparjya, T. Badapanda, S. Behera, B. Behera, P.R. Das, Phase Trans 93, 245–262 (2020)
B. Tiwari, R.N.P. Choudhary, J. Alloys Compd. 493, 1–10 (2010)
T. Rhimi, G. Leroy, B. Duponchel, K. Khirouni, S. Guermazi, M. Toumi, Ionics 24, 1305–1312 (2018)
C. Behera, P. Nayak, R.N.P. Choudhary, Mater. Res. Bull. 43, 401–410 (2008)
T. Mondal, B.P. Majee, S. Das, T.P. Sinha, T.R. Middya, T. Badapanda, P.M. Sarun, Ionics 23, 2405–2416 (2017)
B.C. Sutar, R.N.P. Choudhary, P.R. Das, Ceram. Int. 40, 7791–7798 (2014)
A.K. Jonscher, Dielectric Relaxation in Solids (Chelsea Dielectrics Press, London, 1983)
R. Padhee, P.R. Das, B.N. Parida, R.N.P. Choudhary, J. Phys. Chem. Solids 74, 377–385 (2013)
D.K. Pradhan, R.N.P. Choudhary, C. Rinaldi, R.S. Katiyar, J. Appl. Phys. 106, 024102 (2009)
M. Bouziane, M. Taibi, A. Boukhari, Mater. Chem. Phys. 129, 673–677 (2011)
A. Manohar, C. Krishnamoorthi, J. Alloys Compd. 722, 818–827 (2017)
V. Purohit, R. Padhee, R.N.P. Choudhary, Ceram. Int. 44, 3993–3999 (2018)
S.C. Panigrahi, P.R. Das, R. Padhee, R.N.P. Choudhary, Ferroelectrics 524, 14–29 (2018)
P. Liang, F. Li, X. Chao, Z. Yang, Ceram. Int. 41, 11314–11322 (2015)
S. Pattanayak, R.N.P. Choudhary, P.R. Das, S.R. Shannigrahi, Ceram. Int. 40, 7983–7991 (2014)
K.J. Hamam, F. Salman, Appl. Phys. A 125, 621 (2019)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saparjya, S., Behera, S., Badapanda, T. et al. Effects of La3+ addition on the phase transition, microstructure, and electrical properties of Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics. J Mater Sci: Mater Electron 31, 8116–8126 (2020). https://doi.org/10.1007/s10854-020-03352-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03352-4