Abstract
Undoped ZnO thin films were deposited on sodocalcic glass substrates by the spray pyrolysis technique. Atomization of the starting solution was made by ultrasonic excitation. The effect of the water content in the starting solution on the structural, optical, and morphological properties of the films was studied. All films showed mirror-like finish with good adherence to the substrate. A preferential (002) growth was observed in all the films, although the increase in the water content leads to a slight increase in the (101), (102), (103) peaks intensity. Surface morphology changes slightly with water content variation in the starting solution, since films deposited with low water content show hexagonal shaped grains with round edges and a wide distribution in the average size; whereas well-defined rod-shaped grains are present in films manufactured from a starting solution with high water content. From photoluminescence, PL, measurements at room temperature it was found that, as the water content in the starting solution increases, both the UV and the green emission increase at a constant deposition temperature of 425 °C. PL measurements as a function of the substrate temperature for the optimal water content show that, at a low temperature the green PL peak intensity shows an increase with the temperature, reaching a maximum at 425 °C; however, at higher temperatures the PL response decreases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zinc oxide (ZnO) in thin film form has attracted the interest scientific due to the wide variety of characteristics, such as simultaneous high conductivity and transparency in the visible spectrum, piezoelectric properties, high chemical stability against reducing environments, and antimicrobial properties, among others. These properties make ZnO attractive for applications in different fields, such as different electronic devices [1,2,3], thin film solar cells [4], catalysis [5], and health [6]. The abundance of the raw material and the low toxicity reported make ZnO an excellent ecological material for different industrial applications [4].
ZnO thin films with desired properties can be deposited by a wide variety of physical and chemical techniques [5]. For transparent electrode applications, ZnO thin films with simultaneous high conductivity and optical transmittance in the visible region can be manufactured at relatively low cost on large areas from different chemical techniques. Among them, sol–gel [7] and chemical spray [8] compete well; however, in the case of sol–gel films it is required an extra step, consisting in a heat treatment at moderate temperature (100–200 °C). This is not the case of chemical spray technique, since good quality films can be directly obtained in as-grown films.
Regarding chemical spray atomization some efforts have been made in order to enhance the quality of the films. Atomization of the solution is a key aspect in this respect, as a narrow distribution in the droplet size can have a beneficial effect in the finish of the films. The pulverization of the solution assisted by ultrasonic excitation (USP) [9] has been established as an economical alternative for depositing high quality films. It is worthy of mention that this atomization route is considered a derivation of pneumatic atomization, based on the classical and well-known drop model of Viguie and Spitz [10]. It has been evidenced that, in ultrasonic atomization, the droplet size depends strongly on the ultrasonic frequency, and hence the droplet size distribution can be narrower than the obtained from the pneumatic technique. As a result, it has been encountered that films deposited by USP technique present a smoother surface and a better crystallinity [11]. Moreover, the overall deposition process cost is reduced, which makes attractive the deposition of high cost materials. In recent years, a lot of work embracing the study of undoped ZnO [12, 13] and doped ZnO thin films [14, 15] about the study of the effect of deposition variables on the physical properties, such as substrate temperature [16, 17], annealing treatments [18], doping and precursor concentrations [19, 20], among others, has been reported. However, due to the high cost of professional equipment, alternative solutions have been suggested, this is the case of the home-made equipments, or commercial and simple humidifiers adapted for laboratory deposition, all of them with good results [21].
Although a great deal of reports have been generated on ZnO thin films deposited by UPS giving rise to a broad knowledge on the effect of deposition variables on the characteristics of ZnO films, further work is still required to reach a complete knowledge on the USP process. In this respect, in this work, we are proposing to study the solvent effect on the characteristics of ZnO films deposited by USP. Some years ago, we reported for the first time the successful deposition of fluorine doped ZnO thin films with low resistivity and high transmittance based on the pneumatic atomization [22]. It should be mentioned that in that case the starting solution required of the addition of acetic acid and water for having a long term chemical stability. As can be confirmed, almost all authors working with USP technique do not use neither acetic nor water as solvent but only methanol. An exception is the interesting work on ZnO by Bouzid et al. [23]. Therefore, we propose to explore the solvent effect on the physical properties of undoped ZnO films.
Then, in this work the effect of water content in the starting solution on the characteristics of undoped ZnO thin films deposited on sodalime glass substrates by USP is reported.
2 Experimental
2.1 Films deposition
ZnO thin films were deposited from 0.2 M starting solutions prepared from zinc (II) acetate ([Zn(O2CCH3)2] from Alfa, 98%) dissolved in a mix of deionized water, acetic acid ([CH3CO2H] from Baker, 95%) and methanol ([CH3OH] from Baker, 98%). Four starting solutions with different water:methanol ratios were prepared, according to the following volume proportions (water:acetic acid:methanol): 25:100:875, 50:100:850, 100:100:800, and 200:100:700 (1 L total volume); these will be referred in the text and figures as 2.5/100, 5/100, 10/100, and 20/100, respectively. It is important to notice that acetic acid content was kept constant. The solution atomization was made by an adapted SUNSHINE commercial ultrasonic nebulizer. ZnO films were deposited on Sodalime glass substrates, with an area 2.5 cm × 4 cm, previously cleaned as described elsewhere [24]. Deposition times were fit by trial and error until the desired thickness, 500 nm, was obtained.
2.2 Characterization of ZnO thin solid films
The film thickness of the deposited films was measured by a KLA profilometer (Tencor model P15 with a resolution of 0.15 nm) on a step formed during the deposition process. The microcrystalline structure of the films was analyzed from X-ray diffractometry by using a PANalytical X’pert Pro XRD diffractometer with the Cu-Kα (λ = 0.154056 nm) radiation, using the θ–2θ technique. A XL FEG/SFEG/SIRION scanning electron microscope (SEM) attached with a Noran analytical system was used for morphological analysis. The optical transmittance spectra, at normal incidence, were obtained by a double-beam Shimadzu 2401 PC spectrophotometer, in the UV–Vis region (350–1000 nm) without glass substrate correction. The photoluminescent properties, PL, of films were studied at room temperature using the 325 nm line of a He-Cd laser as excitation source (power: = 0.5 mW); the collected spectrum were analyzed with a half-meter monochromator Sciencetech 9040 and a photomultiplier PMH-02.
3 Results
3.1 Structure
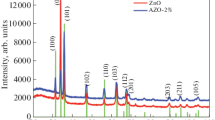
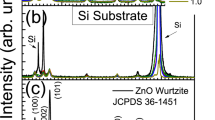
Figure 1 shows the X-ray diffraction spectra of ZnO samples deposited at 425 °C from starting solutions with the four different water contents used, labeled as 2.5/100, 5/100, 10/100, and 20/100. As can be seen from the spectra, the samples are polycrystalline and all peak positions match well with the JPDS standard card for ZnO with hexagonal wurtzite structure [25]. (002) preferential growth was observed in all the cases, irrespective of water content in the starting solution. This result is consistent with other reports [15, 20, 26]. As the water content in the solution increases a slight increase in the intensity of (101), (102), (103) peaks occurs. The mentioned (h k l) peaks are observed at 34.42, 36.32, and 47.62°.
The average crystal size, D, of the films was estimated from the Scherrer equation, D = 0.9λ/Bcosθ, where λ is the X-ray wavelength from Cu (0.15405 nm), θ is the diffraction angle, and B is the full width at half maximum (FWHM). The most intense peak in the spectra, (002) presented at 2θ = 34.42°, was used to calculate the crystal size. The values were around 40 nm in all cases.
3.2 Morphology
Figure 2a–c shows typical SEM micrographs of ZnO thin films deposited at 425 °C from three starting solutions with different water contents in the starting solution; (a) 2.5/100, (b) 10/100, and (c) 20/100. The images show that the water content modifies slightly the surface morphology, so the grain size slightly increases with this. The homogeneity of the morphology is evident and the apparition of voids or porous is clearly visible. Apparently the porosity increases with the water content. All surfaces are covered by regular hexagonal column rods that are almost perpendicular to substrate, indicating that ZnO rods preferentially grow along the (002) planes, as was confirmed by the XRD spectra (see Fig. 1). Unfortunately the resolution of SEM images presented is poor enough to observe fine details; however, by a zoom it is possible to distinguish the size and shape of rods.
Figure 2a shows the morphology of a typical sample deposited from a starting solution with a water content of 2.5/100. It can be observed a surface covered of hexagonal rods with an average diameter size around 250 nm. The increase in the water content in the starting solution leads to both, an improvement of the definition of the grains, that is, the formation of well-defined hexagonal rods (see the squares into Fig. 2b), and a slight increase of the grains size at average sizes around 300 nm. Further increase in water content keeps the grain size constant, but more voids can be evidenced, that is, the distance among the rods seems to be bigger (Fig. 3c). From the obtained results, the influence of the water content on the growth kinetic can be understood if we consider the first stage of the films growth where the formation of nuclei occurs. The nuclei number formed on the substrate is dependent on the evaporation temperature of the solvents, then considering that the acetic acid volume was constant in all the starting solutions, the evaporation temperature of the solution will depend on the methanol/water ratio, then, as the evaporation temperature of methanol (~ 65 °C) is lower than that of water (~ 100 °C), is the water content that influences more the deposition process. As a result, the higher water content less number of nuclei but with bigger size will be obtained.
Similar results were reported by Krunks et al. [27] for ZnO films deposited by pneumatic spray pyrolysis by using ZnCl2 as Zn precursor. However, they presented a study about the effect of the molar concentration of the starting solution and the substrate temperature. Then, as was expected, this result confirms that the growth kinetic of crystals depends of both solution and deposition variables.
3.3 Optical properties
All deposited ZnO thin films were uniform but opaque at naked eye, with a milky appearance. In general, the average optical transmission of the ZnO films was around 50%, irrespective of the water content in the starting solutions. Figure 3 shows the transmittance spectrum of the 2.5/100 ZnO sample. It is worthy to state that films with similar film thickness showed very similar transmittance in the visible region.
Figure 4 shows the evolution of the PL response, measured at room temperature, of ZnO thin films deposited at 425 °C with different water content in the starting solution. From spectra it can be observed the presence of two main emissions, one is weak and located at UV region and the second one is a strong, very broad, in the green range. The spectra shows that as the water content in the starting solutions increase the corresponding intensity of green PL peaks increases as well, reaching a maximum for the samples deposited with the highest water content (20/100). The same trend is also observed for the UV PL, although in this case the intensity is lower than in the case of visible luminescence.
In order to analyze the influence of the substrate temperature on the PL response, Fig. 5 shows the PL spectra of ZnO samples with the highest water content, ZnO-20/100, and deposited at different substrate temperatures, 400, 425, 450, and 475 °C. From the spectra it can be observed that, initially at low deposition temperatures the PL intensity increases with the temperature, reaching a maximum for samples deposited at 425 °C; however, further increase in the substrate temperature shows a decrease in the PL intensity.
According to some authors [23], the broad green band may be originated from the recombination of the photoexcited holes with electrons located in the single ionized oxygen vacancies (Vo), i.e., visible luminescence bands from ZnO are associated with Vo in ZnO thin films. It has also been stated that, as the stoichiometry is reached, that is, when zinc and oxygen balance in the films appears, decreasing the defects due to non-stoichiometry, the visible PL spectrum should decrease dramatically and in the limit, be free from visible emission. Accordingly, the spectra of PL of the films deposited with aqueous solvent in this work, shows that a significant density of Vo are always present into the ZnO lattice even when water supplies oxygen for the ZnO synthesis. It is possible that water excess be also responsible for the low intensity of UV PL peak. On the other hand, stoichiometry deviation is a requisite for the manufacturing of high conductivity films. Under our deposition conditions, it can be expected that doping can be successful.
4 Conclusions
Uniform, opaque at naked eye with a milky appearance, and adherent ZnO thin films were deposited on sodalime glass substrates at different deposition temperatures by the ultrasonic spray pyrolysis technique. The starting solutions contained water at different concentrations. The ZnO thin films showed a (002) preferential growth. Water content commands shape of the grains, as a change from round, almost hexagonal to rod-like geometry was observed due to the increase in the water content in the starting solution. The presence of water during the synthesis process does not influence the crystallinity of the films in a significant way, but increases the PL in the visible region, in the case of samples deposited with 200 ml/L water content. The non-stoichiometric character of the films opens the possibility for successful doping, in order to manufacture good quality transparent electrodes.
References
E. Fortunato, A. Gonçalves, A. Pimentel, P. Barquinha, G. Gonçalves, L. Pereira, I. Ferreira, R. Martins, Appl. Phys. A 96, 197 (2009)
H. Hosono, Thin Solid Films 515, 6000 (2007)
K. Ramamoorthya, K. Kumara, R. Chandramohan, K. Sankaranarayanan, Mater. Sci. Eng., B 26, 1 (2006)
C.G. Granqvist, Sol. Energy Mater. Sol. Cells 91, 1529 (2007)
J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang, S. Jiazhong, Sol. Energy Mater. Sol. Cells 90, 1773 (2006)
R. Dastjerdi, M. Montazer, Colloids Surf. B 79, 5 (2010)
L. Znaidi, Mater. Sci. Eng. B 174, 18 (2010)
P.S. Patil, Mater. Chem. Phys. 59, 185 (1999)
Y. Lee, H. Kim, Y. Roh, Jpn. J. Appl. Phys 20, 2423 (2001)
J.C. Viguié, J. Spitz, J. Electrochem. Soc. 122, 585 (2001)
J. Xu, H. Wang, L. Yang, M. Jiang, S. Wei, T. Zhang, Mater. Sci. Eng. B 167, 182 (2010)
V.V. Kireev, L.N. Dem’yanets, L.E. Li, V.V. Artemov, Inorg. Mater. 46, 154 (2010)
B. Ergin, E. Ketenci, F. Atay, Int. J. Hydrog. Energy 34, 5249 (2009)
M.S. Tokumoto, A. Smith, C.V. Santillia, S.H. Pulcinelli, A.F. Craievich, E. Elkaim, A. Traverse, V. Briois, Thin Solid Films 416, 284 (2002)
T.Y. Ma, S.C. Lee, J. Mater. Sci. Mater. Electron. 11, 305 (2000)
U. Alver, T. Kılınç, E. Bacaksız, S. Nezir, Mater. Chem. Phys. 106, 227 (2007)
M.H. Choi, T.Y. Ma, Influence of substrate temperature on ultraviolet emission of ZnO films prepared by ultrasonic spray pyrolysis. J Mater. Sci. 41, 431 (2006)
J.-H. Lee, B.-W. Yeo, B.-O. Park, Effects of the annealing treatment on electrical and optical properties of ZnO transparent conduction films by ultrasonic spraying pyrolysis. Thin Solid Films 457, 333–337 (2004)
I. Akyuz, S. Kose, F. Atay, V. Bilgin, The optical, structural and morphological properties of ultrasonically sprayed ZnO:Mn films. Semicond. Sci. Technol. 21, 1620–1626 (2006)
L.A. Patil, A.R. Bari, M.D. Shinde, V. Deo, M.P. Kaushik, Effect of precursor concentrations on structural, microstructural and optical properties of nanocrystalline ZnO powder synthesized by an ultrasonic atomization technique. Phys. Scr. 82, 035601 (2010)
A. Djelloul, K. Bouzid, F. Guerrab, Role of substrate temperature on the structural and morphological properties of ZnO thin films deposited by ultrasonic spray pyrolysis. Turk. J. Phys. 32, 49–58 (2008)
A. Guillén-Santiago, M.L. Olvera, A. Maldonado, R. Asomoza, D.R. Acosta, Phys. Status Solidi (A) 201(5), 952 (2004)
K. Bouzid, A. Djelloul, N. Bouzid, J. Bougdira, Electrical resistivity and photoluminescence of zinc oxide films prepared by ultrasonic spray pyrolysis. Phys. Status Solidi (A) 206(1), 106 (2009)
M. Avendaño-Alejo, C. Márquez-Beltrán, H. Gómez, J. Vega-Pérez, M.L. Olvera, L. Castañeda, A. Maldonado, A. Escobedo-Morales, Mater. Sci. Semicond. Process. 14, 114 (2011)
Joint Committee on Powder Diffraction Standards (JCPDS), File Number: 00-020-1435
M.H. Choi, T.Y. Ma, J. Mater. Sci. 41, 431 (2006)
M. Krunks, T. Dedova, I.O. Acik, Thin Solid Films 515, 1160 (2006)
Acknowledgements
The technical support of M. A. Luna-Arias and A. Tavira-Fuentes is thanked. CONACyT support is also gratefully acknowledged under contract 80502.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maldonado, A., Acosta, D.R. & Olvera, M.L. Undoped ZnO thin films deposited by ultrasonic spray technique: effect of the water content in the starting solution on the physical properties. J Mater Sci: Mater Electron 31, 6525–6529 (2020). https://doi.org/10.1007/s10854-020-03274-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03274-1