Abstract
Spherical Gd2O3:Er3+ and Gd2O3:Er3+ @ silica nanoparticles have been successfully synthesized by a multistep procedure including precipitation and sol–gel processes from rare-earth nitrates, TEOS and urea as starting precursors. The structure, morphology and chemical composition of the products were characterized by X-ray diffraction, field emission scanning electron microscopy, and fourier transform infrared spectroscopy. The results indicated that the obtained phosphors consist of separated, non-agglomerated spheres in nanoscale with an uniform, ideal spherical shape. The suitable amount of TEOS was addressed to obtain the spherical Gd2O3:Er3+ @ silica nanocomposites with the luminescence intensity almost unchanged with respect to the uncoated Gd2O3:Er3+ sample. Besides, the upconversion emission spectra showed intense green and red emission bands under 980-nm laser diode excitation with remarkable increase in the red emissions. The upconversion luminescence followed the two-photon mechanism for green and red emissions. With obtained results, the prepared nanospheres are expected to be a potential material to create functional groups for subsequent bio-conjugation with various biomolecules in medical diagnostics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rare-earth ion (RE)-doped inorganic luminescent nanomaterials are important and interesting subjects for fundamental research and applications in many fields of technology. Among these materials, Gd2O3:Er3+ is widely known as a high-efficient oxide phosphor and attracted much attention of researchers worldwide. Gd2O3 is usually used as a host matrix for doping with various rare-earth ions [1]. Er3+ ion is an attractive activator for achieving intense emission in green and red spectral regions [2], especially for preparation of converting luminescence materials [3, 4]. Owing to these advantages, Gd2O3:Er3+ nanoparticles have been intensively investigated and found a wide range of applications. Several methods have been applied for synthesis of the Gd2O3 nanoparticles (NPs), undoped or doped with RE ions. Many interesting systems of nanomaterials have also been investigated for different application purposes [5,6,7]. Besides that, the energy levels of Er3+ ion in Gd2O3 matrix, the energy transfers in Er3+-doped Gd2O3 NPs, as well as the luminescence conversion effect, color tunability of Gd2O3, Gd2O3:Er3+ and Gd2O3:Er3+/Yb3+ were the subjects of many investigations [8,9,10].
Recently, much effort has been made in term of the usage of the Ln3+-doped fluorescent compounds in biomedicine based on their excellent optical properties, such as sharp emission lines, long lifetimes and photo-stability [11, 12]. For this purpose, a perfect spherical shape, narrow size distribution, and non-agglomeration of material are required. Under these conditions, the phosphor shows high brightness, high-resolution, and low-light scattering [13]. So far, many synthetic routes focused on controlling the spherical shape, size and distribution of phosphor particles, such as hydrothermal synthesis of Y2O3:Yb3+/Er3+ nanospheres with upconversion luminescence [13], aging at elevated temperature for preparation of monodispersed colloidal particles of lanthanide compounds [14], chemical precipitation for preparation of core–shell Gd2O3:Eu @ mSiO2 hollow nanospheres for drug release [15, 16], sol–gel process for creating SiO2 layer onto the surface of nanophosphor [17, 18], etc. Although many efforts have been made, to our knowledge, the synthesis of nanospheres with controllable shape and size and their properties has been relatively less studied in comparison with the nanopowder materials fabricated by solid-state reaction or combustion methods. In addition, the surface modification and functionalization are always needed to make the phosphor useful for further application. Silica [SiO2−x(OH)x] is an excellent candidate for modifying the surface of phosphors because of its high dispersion. The OH groups on silica matrix give the surface hydrophilic properties, make it better dispersed in different solvents. Then, the structure can be easily functionalized to develop high-resolution optical imaging in biomedical diagnose. In our previous publication [19], we have reported the optical properties of Gd2O3:Er3+ nanopowders fabricated by EDTA-assisted combustion method and their composite structures formed by coating the NPs with silica.
To continue this research, we focused on Gd2O3:Er nanosphere in this work. We applied with great modify the chemical precipitation method for synthesis of uniform Gd2O3:Er3+ NSPs followed by the coating Gd2O3:Er3+ with silica using the sol–gel method to obtain Gd2O3:Er3+ @ SiO2 nanospheres. This method is simple, low cost, high efficiency and environmental friendly. Using an appropriate synthesis procedure following a careful stepwise heat-treating regime we intend to obtain Gd2O3:Er3+ and Gd2O3:Er3+ @ silica nanospheres with sufficient luminescent and structural properties, so that can be useful for application in medical field. The crystalline phase identification, morphology, chemical composition and luminescence properties of the products have been investigated and discussed.
2 Experimental details
2.1 Materials
The initial chemicals including Gd(NO3)3·6H2O 99.9% (Sigma-Aldrich), Er(NO3)3·5H2O 99.9% (Sigma-Aldrich), TEOS [(C2H5O)4·Si] 99.9% (Sigma-Aldrich), urea [CO (NH2)2] 99.8% (Prolabo), and HNO3 65 wt%, NH4OH 28 wt%, absolute C2H5OH (all with purity of A.R) were used without further purification.
2.2 Synthesis of Gd(OH)CO3·H2O:Er3+ and Gd2O3:Er3+ nanospheres
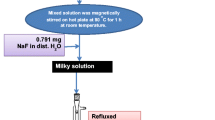
To synthesize the Gd2O3:Er3+ nanospheres, the Gd(OH)CO3·H2O):Er3+ NSPs were first prepared via homogeneous precipitation. Urea served as a precipitation agent because it can self-decomposes into the OH− and CO32− at temperature of about 83 °C [16, 20]. It is a simple and often been used method for preparation of lanthanide oxide phosphors. In a typical synthesis, the stoichiometric volume of 0.4 M Gd(NO3)3·6H2O and 0.1 M Er(NO3)3·5H2O solutions in DI water were mixed in a 150 ml glass bottle and stirred during 15 min to form homogeneous and clear solution. A necessary amount of urea, calculated from the selected molar ratio of the amount of urea and total amount of rare-earth ions, denoting as urea/[Gd3+ + Er3+] molar ratio, was dissolved in 100 ml DI water, then the mixed RE solution was added dropwise to the urea solution by continuous stirring in 2 h at room temperature. The pH of the reaction solution was adjusted and kept at 6.5 using NH4OH solution during the reaction time. The temperature of the reaction solution was then slowly raised up to 85 °C and kept constant for 2.5 h in an oil bath. The formation of Gd(OH)CO3·H2O:Er3+ occurred according to the following reaction:
After the precipitation was completed, the reaction solution was quickly cooled down to room temperature and then has been centrifuged to separate the precipitate. The precipitate was washed with ethanol and DI water, dried in air at 70 °C for 24 h, at 105 °C for 2 h and at 150 °C for 2 h. In these synthetic experiments, the concentration of Er3+ ion was 1.5, 1.8 and 2.0 mol%. The optimized urea/[Gd3+ + Er3+] molar ratio of 25/1 was selected according to previous experience [21]. The reaction products were dry white spherical Gd(OH)CO3·H2O):Er3+ particles. One part of the product had undergone a stepwise sintering process at 200 °C for 2 h, at 400 °C for 2 h, at 600 °C for 2 h and at 650 °C for 3 h resulting in the formation of Gd2O3:Er3+ oxide spheres [16, 20, 21].
The other part served as starting material for coating with silica shell.
2.3 Synthesis of Gd(OH)CO3·H2O):Er3+ @ silica and Gd2O3:Er3+ @ silica nanospheres
Similar to the synthesis of Gd2O3:Er3+ NSPs, the synthesis of Gd2O3:Er3+ @ silica NSPs consists two steps: The first step is preparation of Gd(OH)CO3·H2O:Er3+ @ silica particles by sol–gel method and the next step is the calcination process to converse Gd(OH)CO3·H2O:Er3+ @ silica into Gd2O3:Er3+ @ silica nanospheres. In the first step, 150 mg of Gd(OH)CO3·H2O:Er3+ were well dispersed in a mixed solution of 150 ml of absolute C2H5OH and 60 ml of DI water by ultra-sonication for 60 min 150 µl of tetraethoxysilane (TEOS) dispersed in 10-ml ethanol was added dropwise, followed by adding 1.0 ml of NH4OH (28 wt%). The solution was then continuously stirred for 24 h at room temperature. After that, the solution was heated to 60 °C and kept for 60 min. Then, it was cooled down to room temperature and the formed particles were separated, washed until pH 7 and dried subsequently at 70 °C for 24 h, at 105 °C for 2 h and at 150 °C for 2 h. The further heat-treating regime included heating at 200 °C for 2 h, at 400 °C for 2 h, at 600 °C for 2 h, and at 650 °C for 3 h, subsequently. The final product was Gd2O3:Er3+ @ silica nanospheres. The same procedure has been applied for the synthesis of the sample with 200-µl TEOS.
2.4 Characterization
The structure and morphology of all samples were investigated using a Bruker D8-Advance diffractometer, and field emission scanning electron microscopy (FESEM, model: Hitachi S-4800). Functional groups were measured with the aid of a fourier transform infrared spectrometer (FTIR, Model Fourier NEXUS 670). The PL spectra and the PL excitation (PLE) spectra of all samples were recorded using a FL 3–22 HORIBA spectrometer with double monochromaters. Upconversion luminescent spectra were measured on an iHR 550(Jobin-Yvon) equipment using 980-nm laser diode (LD) excitation.
3 Results and discussions
3.1 Structure and morphology
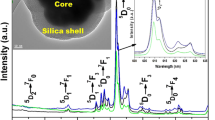
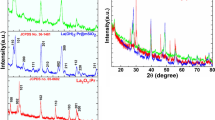
The XRD analysis reveals that the as-prepared Gd(OH)CO3·H2O):Er3+ nanospheres after drying at 200 °C is amorphous (figure not shown). This result is coincident with the previous reports [16, 20]. Thermal decomposition via calcinations at 650 °C for 3 h results in the conversion of amorphous Gd(OH)CO3·H2O:Er3+ to Gd2O3:Er3+ crystalline material. The XRD pattern pointed out the pure cubic phase of gadolinium oxide (JCPDS Card No. 43-1014), as shown in Fig. 1a. Besides that, the broadening of XRD peaks was observed suggesting the formation of nano-sized crystallites. Using Scherrer formula, the crystallite size of these nanocrystals was calculated to be 40 nm in average. Moreover, FESEM image of Gd(OH)CO3·H2O:Er3+ nanospheres is shown in Fig. 1b. It indicated that the as-prepared Gd(OH)CO3·H2O:Er3+ nanospheres have spherical shape with smooth surface and homogeneously monodispersed. The grain size estimated from FESEM image ranges from 200 to 250 nm. After calcinations at high temperature (650 °C for 3 h), amorphous Gd(OH)CO3·H2O:Er3+ nanospheres were thermal decomposed and Gd2O3:Er3+ crystalline phase was formed instead [13]. The spherical shape was remained, as shown in Fig. 2a. It was the result of our careful stepwise heat treatment regime. After being annealed at 650 °C for 3 h, the average spheres size reduced remarkably to 80 nm of uncoated Gd2O3:Er3+ sphere, estimated from FESEM image (Fig. 2a), and is almost twice the crystallite size determined from XRD data. This discrepancy can be explained by the fact that the spherical grains observed in FESEM image might comprised of smaller crystallites, similar to the case reported in Ref.[1]. Figure 2b gives the FESEM image of Gd2O3:Er3+2 mol% @ silica (200 µl TEOS) obtained after calcining the Gd(OH)CO3·H2O:Er3+2 mol% @ silica (200 µl TEOS) NSPs at 650 °C for 3 h. One can see that the spherical core Gd2O3:Er3+ has been homogeneously coated with a silica layer. The reason for the easy reaction of TEOS with the core surface to form homogeneous silica layer is the hydrated phase and the presence of OH– groups in sol–gel process [20]. The average size of Gd2O3:Er3+2 mol% @ silica spheres estimated from FESEM image was about 130 nm.
3.2 Chemical composition
FTIR spectroscopy was performed to qualitatively investigate the chemical composition and chemical bonding of the products. The FTIR spectra of the uncoated and silica-coated Gd2O3:Er3+ 2 mol% samples are shown in Fig. 3. In the case of uncoated Gd2O3:Er3+ NSPs the bands at around 545, 688 and 757 cm−1 can be assigned to the Gd–O vibration of gadolinium oxide and the peak at 1633 cm−1 probably corresponds to the Er–O vibration [2, 19, 22]. This confirmed the formation of the Er3+-doped Gd2O3 nanocrystals. In the FTIR spectra of silica-coated Gd2O3:Er3+ 2 mol% NSPs an additional characteristic absorption band of Si–O–Si group was clearly seen around 1082 cm−1 [23], which confirmed the formation of Gd2O3:Er3+ @ silica structure. The band at approximately 3400 cm−1 revealed the presence of OH− groups from surface absorbed H2O and hydroxide molecules.
3.3 Optical properties
Photoluminescence behavior of the samples have been investigated to elucidate the energy levels and transitions of Er3+ ions in Gd2O3 matrix. The photoluminescence excitation spectrum (PLE) of prepared Gd2O3:Er3+ 2 mol% NSPs annealed at 650 °C for 3 h, monitoring at λem = 562 nm (shown in Fig. 4a) indicates that one can use the wavelengths of 275 or 379 nm for excitation of the Gd2O3:Er3+ nanophosphors. The strongest peak was at 379 nm. Figure 4b.1 presents the photoluminescence emission spectrum (PL) of the same sample under 275-nm excitation. This excitation activated the 8S7/2 to 6I7/2 state of Gd3+.ion. The spectrum consists characteristic emission bands of Er3+ ion in the blue-green, green and red spectral regions, corresponding to the transitions from Er3+2H9/2 → 4I15/2 (409 nm), 4F7/2 → 4I 15/2 (483 nm), 2H11/2 → 4I15/2 (542 nm), 4S3/2 → 4I15/2 (553 and 562 nm) and 4F9/2 → 4I15/2 (around 661 nm), respectively. The strongest peak positioned at 553 nm. According to the energy level diagram of Er3+ ions in Gd2O3:Er3+NPs (Fig. 7a), by absorption of the UV light of 275 nm the ground state of Gd3+ ion 8S7/2 excites to 6I7/2 energy state. The 6I7/2 state converted 6P7/2 by non-radiative decay. Then, the energy from 6P7/2 level of the Gd3+ ion transferred to the 2P3/2 level of the nearby Er3+ ion and non-radiatively transferred to the lower levels of the Er3+ ion, giving the visible emissions of the phosphor [24]. The emission spectrum of Gd2O3:Er3+ 2 mol% NSPs under 379-nm excitation was shown in Fig. 4b.2. The emissions correspond to the transitions of Er3+ energy levels, namely, 2H9/2 → 4I15/2 (409 nm), 2H11/2 (522, 538 nm), 4S3/2 (548, 553, 562 nm) and 4F9/2 (around 661 nm) to the ground energy state 4I15/2 were observed. The results of PL analysis show that the prepared samples exhibit high-luminescence efficiency. We also noted that the Gd2O3: Er3+ 1.8 mol% sample exhibits the higher value of luminescent intensity than those of the others and will be used for further investigations.
As mentioned above, the spherical Gd2O3:Er3+ @ silica composite structure has been achieved by means of homogenous precipitation combined with sol–gel method using TEOS as silica source. The sol–gel method is well known as a main synthesis technique for creating a silica layer deposited on the surface of nanomaterials to modify, protect their surface and enhance the quality of nanophosphors. The TEOS concentrations of 150 µl and 200 µl were used based on previous experience [21]. Figure 5 presents the PL spectra of uncoated Gd2O3:Er3+ 1.8 mol% after 3 h of heating at 650 °C and of Gd2O3:Er3+ 1.8 mol% coated by 150 µl and 200 µl TEOS, heated for 3 h at 650 °C, recorded at room temperature, excited by 379-nm wavelength. We can see that the spectra of Gd2O3:Er3+1.8 mol% @ silica display similar emission bands to uncoated Gd2O3:Er3+, which indicated the similar transitions process of Er3+ ion in Gd2O3 host matrix. The emission intensities of coated samples slightly decreased. That can be explained by comparing the above—mentioned XRD patterns of Gd2O3:Er3+ and Gd2O3:Er3+ @ silica samples. Accordingly, the XRD patterns show that both samples after heating at 650 °C for 3 h remained the cubic crystal structure, so this reduction should be due to the effect of the silica layer deposited onto the surface of NSPs and probably related to the presence of more OH group in coated samples [25]. It may have raised defects on the surface of Gd2O3:Er3+ nanoparticles. Our results are in good agreement with observations previously reported [15, 17]. It is worthy to point out from Fig. 5 that the sample coated with 150-µl TEOS gives a luminescence intensity nearly that of the uncoated sample. It means that we selected a suitable SiO2 thickness to hinder the decrease of PL intensity of sample. Although the silica coating of the surface of materials may cause a slight decrease in their luminescence intensity, however, luminescent nanomaterials with perfect spherical shape, coated by silica with an appropriate thickness are always preferred for many applications, especially in biomedicine as a targeting drug-delivery structures, etc.
Upconversion luminescence: Fig. 6a presents the emission spectra of the Gd2O3:Er3+ 1.8 mol% (red curve) and Gd2O3:Er3+1.8 mol% @ silica (150 µl TEOS) (blue curve), annealed stepwise up to 650 °C for 3 h recorded in the range of 450–750 nm, under 980-nm LD excitation. The spectrum show a strong red light, including many bright red emissions of Er3+ near 661 nm, corresponding to Er3+4F9/2 → 4I15/2 transition together with strong green emission bands centered at 525, 558 and 563 nm assigned to Er3+2H11/2 → 4I15/2, 4S3/2 → 4I15/2 transitions, respectively. The UCL spectra of silica-coated Gd2O3:Er3+ 1.8 mol% sample is almost the same as that of Gd2O3:Er3+ 1.8 mol% uncoated sample, except a little lower intensity, which should be ascribed to silica coating. It is worth to note the remarkable enhancement of the red emissions in both Gd2O3:Er3+ @ silica or uncoated samples obtained by the procedure described above. They are stronger in comparison with the red emissions of Gd2O3:Er3+ nanopowder obtained by the combustion method [19]. This phenomenon may relate to the location of Er3+ and the population of the 4F9/2 red-emitting levels [13]. The strong red emission materials provide various application possibilities in biomedicine and biosensors. The dependence of the emission intensities on the laser diode pump power for the red and green emissions under 980-nm excitation in Gd2O3:Er3+ 1.8 mol% nanospheres is shown in Fig. 6b. The intensity of the emission bands linearly related to the pump power. The calculated values of slope ‘n’ indicated that the upconversion process of the samples was two photons transitions for green and red emissions. More clearly, Fig. 7 presents the observed energy levels, excitation and emission transitions of all investigated samples. All the emission bands are assigned to the transition between the energy levels of the Er3+ ion. Any pair of energy levels in Fig. 7 with closely matching energies bears a potential for energy transfer.
4 Conclusions
Using a multistep synthesis procedure, the monodispersed spherical Gd2O3:Er3+ and Gd2O3:Er3+ @ silica nanoparticles (NSPs) were obtained. The employing of a careful stepwise heating procedure ensures to obtain the products with perfect spherical shape. Both uncoated and silica-coated Gd2O3:Er3+ nanospheres exhibited not only downconversion but also upconversion luminescence properties. Especially, with the tetraethoxysilane amount of 150 μl, the luminescence intensity of the spherical Gd2O3:Er3+ @ silica nanocomposites is comparable to that of the uncoated Gd2O3:Er3+ sample. Possessing ideal spherical shape, strong luminescence emissions, especially high red upconversion emission, the synthesized Gd2O3:Er3+-based luminescent nanoparticles can be subjected to further studies in many fields, especially in sensing and biomedicine.
References
I. Kaminska, D. Elbaum, B. Sikora, P. Kowalik, J. Mikulski, Z. Felcyn, P. Samol, T. Wojciechowski, R. Minikayev, W. Paszkowicz, W. Zaleszczyk, M. Szewczyk, A. Konopka, G. Gruzeł, M. Pawlyta, M. Donten, K. Ciszak, K. Zajdel, M. Frontczak-Baniewicz, P. Stępień, M. Łapiński, G. Wilczyński, K. Fronc, Nanotechnology 29, 025702 (2018)
Y.A. Kuznetsova, A.F. Zatsepin, V.A. Mashkovtsev, V.N. Rychkov, IOP Conf. Ser. J. Phys. Conf. Ser. 917, 052015 (2017)
K. Zheng, Z. Liu, Y. Liu, W. Song, W. Qin, J. Appl. Phys. 114, 183109 (2013)
X. Chen, E. Ma, G. Liu, J. Phys. Chem. C 111, 10404 (2007)
G. Boopathi, S.G. Raj, G.R. Kumar, R. Mohan, Proc. Mater. Sci. 6, 1436 (2014). https://doi.org/10.1016/j.mspro.2014.07.123
Y.A. Kuznetsova, A.F. Zatsepin, I.O.P. Conf, Ser. J. Phys. Conf. Ser. 917, 062001 (2017)
R.K. Tamrakar, D.P. Bisen, K. Upadhyay, I.P. Sahu, M. Sahu, RSC Adv. 6, 92360 (2016)
R.K. Tamrakar, D.P. Bisen, N. Brahme, K. Upadhyay, Optik 126, 2654 (2015)
R.K. Tamrakar, D.P. Bisen, K. Upadhyay, I.P. Sahu, J. Alloys Compd. 655, 423 (2016)
M. Pokhrel, G.A. Kumar, D.K. Sardar, J. Mater. Chem. A 1, 11595 (2013)
M.T. Man, H.S. Lee, Sci. Rep. 9, 4613 (2019)
E. Hemmer, H. Takeshita, T. Yamano, T. Fujiki, Y. Kohl, K. Love, N. Venkatachalam, H. Hyodo, J. Mater. Sci. Mater. Med. 23, 2399 (2012)
Y. Yang, J. Gu, R. Yang, Q. Shang, J. Yang, Nanosci. Nanometrol. 2, 41 (2016)
E. Matijevic, W. Hsu, J. Colloid, Interface Sci. 118, 506 (1987)
R. Li, L. Li, Y. Han, S. Gai, F. He, P. Yang, J. Mater. Chem. B. 2, 2127 (2014)
W. Di, X. Ren, L. Zhang, C. Liu, S. Lu, Cryst. Eng. Commun. 13, 4831 (2011)
K.M. Lin, C.C. Lin, Y.Y. Li, Nanotechnology 17, 1745 (2006)
H. Xiao, P. Li, F. Jia, L. Zhang, J. Phys. Chem. C 113, 21034 (2009)
L.Q. Minh, T.K. Anh, N.D. Hung, P.T.M. Chau, N.T.Q. Hai, H.V. Tuyen, V.T.T. Ha, V.D. Tu, W. Strek, J. Rare Earth 37, 1126 (2019)
W. Song, W. Di, W. Qin, Dalton Trans. 45, 7443 (2016)
T.K. Anh, N.T. Huong, P.T. Lien, D.K. Tung, V.D. Tu, N.D. Van, W. Strek, L.Q. Minh, Mater. Sci. Eng. B 241, 1 (2019)
S.K. Ranial, A.K. Soni, V.K. Rai, Methods Appl. Fluorsc. 5, 035004 (2017)
A. Vosk, A. Saar, Nanoscale Res. Lett. 9, 47 (2014)
R.K. Tamrakar, D.P. Bisen, K. Upadthyay, N. Brame, Superlattice Microstruct. 81, 34 (2015)
S. Xu, D. Fang, Z. Zhang, Z. Jiang, J. Solid State Chem. 178, 2159 (2005)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hai, N.T.Q., Anh, T.K., Chau, P.T.M. et al. Multistep synthesis and upconversion luminescence of spherical Gd2O3:Er and Gd2O3:Er @ silica. J Mater Sci: Mater Electron 31, 3354–3360 (2020). https://doi.org/10.1007/s10854-020-02883-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-02883-0