Abstract
This work presents the synthesis and characterization of nanostructured cadmium sulfide thin films obtained by an ammonia-free chemical bath deposition process. Different levels of Cu ion doping were achieved by the ion exchange method of CdS at room temperature. The main objective was to control the energy band gap adjusting the copper concentration and to gather information about Cu-doped CdS thin films. Optical absorbance measurements were performed to obtain the band gap value through Tauc’s plot method. XPS measurements were used to obtain the copper and cadmium concentration. In addition, SEM micrographs were collected to obtain information about surface and structural arrangement on Cu-doped CdS thin films. It was also found that the exchange of cadmium by Cu-ions promoted the formation of a hexagonal crystalline structure, which was different than the initial pure cubic structure. Through optical characterization, it is possible to evaluate the impact of the metallic dopant on the band gap value, finding that it can be controlled by the percentage of added copper, which opens the possibility of applying this material to the development of electronic devices such as field effect transistors. In addition, a prototype of a MOS structure was fabricated employing both the undoped and the Cu-doped CdS thin film to corroborate its possible application as a field effect transistor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is known that the study of II–VI semiconductors has generated a lot of attention in the material science community over the pasts decades due to the possibility of structural and optical modifications given by the quantum confinement effect [1, 2] and the very interesting properties achieved by the metallic ion doping [3,4,5]. One of the most attractive II–VI-type semiconductors is cadmium sulfide (CdS), since it is a n-type photo-sensible semiconductor, with a direct band gap value of 2.4 eV at room temperature. Many research groups have been investigating this material due to its optical and opto-electronical properties, which allow its use in applications such as solar cells [6, 7], thin film transistors [8, 9], photo diodes [10] and light-emitting diodes (LED) [11, 12]. CdS crystals can be grown in two distinct structural phases: cubic (Zinc-blende) and hexagonal (wurtzite) [13]. In addition, CdS thin films can be produced by different techniques including: sol–gel [14], close spaced vapor transport (CSVT) [15], pulsed laser deposition (PLD) [16], RF sputtering [17], chemical vapor deposition (CVD) [18, 19], spray pyrolysis (SP) [20, 21] and chemical bath deposition (CBD) [7, 8, 22]. The latter technique was chosen in the present work due to the fact that it does not require high vacuums nor high temperatures so there is no emission of toxic gases [23]. Chemical bath deposition employs a controlled chemical reaction to deposit nanostructured thin films through a complexing agent [24]. It is worth to mention that typical chemical bath deposition of CdS thin films employs ammonia as complexing agent for the cadmium ions in the reaction solution. It is evident that the large-scale fabrication of CdS thin films, employing great amounts ammonia, could rise some serious environmental issues due to its high volatility and toxicity. Furthermore, the high volatility of ammonia could produce unwanted variations on the pH of the reaction solution in the deposition process, leading to difficulties in the reproducibility of the films. Due to these issues, there is a consistent effort in the search for alternative complexing agents to ammonia in order to make the CBD process more environmentally friendly [25,26,27,28]. The films can be grown in large areas with good reproducibility [29] making CBD a feasible technique for mass production. Since CdS is a promising material for application in solar cells, it has been doped with several materials such as gallium [30], indium [31], boron [32], manganese [33], tin [34], sodium [35], silver [18, 36] and copper [21, 37, 38]. In particular, copper doping has gained our attention since it is capable to change the properties of CdS in a relatively feasible way, increasing its resistivity in dark, but at the same time increasing its conductance in the presence of light, which means more photo-sensibility due to the increase in carrier Cu ions. In addition, it is also known that copper doping changes the CdS n-type semiconductor to a p-type semiconductor [39]. The present work focuses on the synthesis and characterization of nanostructured CdS thin films obtained by an ammonia-free chemical bath deposition process, where different levels of Cu2+ ion doping were achieved by the ion exchange method. This doping technique involves the exchange of ions between an electrolyte solution (aqueous phase) and similarly charged ions (cations in the Cu2+ doping) immobilized in an ion exchange material (solid phase) that takes place through a stoichiometric reversible reaction [40]. In this technique, the exchange is produced only between cations while leaving the anionic part of the lattice intact [41]. In contrast, in the in situ doping technique, there is a probability to produce stronger structural changes during the growth process since the dopant is present in the precursor solution [42, 43]. In addition, the ion exchange process is driven by diffusion, hence, the Cu2+ doping ions will be present mostly on the surface of the thin films [44]. Trough optical characterization, it is possible to evaluate the impact of the metallic dopant on the band gap value.
2 Experimental
The CdS and Cu-doped CdS thin films were deposited by the chemical bath deposition method in soda lime glass substrates. The fabrication method consists of a five steps process: cleaning, deposition, a second cleaning, doping and annealing treatment. All substrates were cleaned three times before deposition; to this end, the substrates were submerged in acetone, isopropyl alcohol and deionized water (one after the other), for 3 min in an ultrasonic bath. Once the cleaning was completed, the deposition process is performed. The chemical bath deposition consisted of an immersion of the substrates on a solution composed by 4 ml of cadmium nitrate 0.1 M, 5 ml of glycine 0.1 M, 2 ml buffer pH 11, 5 ml of thiourea 1 M and 44 ml of deionized water at 70 °C for 25 min. The samples were then cleaned with deionized water for 5 min in an ultrasonic bath. After the second cleaning, the produced CdS films were dipped in a solution comprising of 60 ml of cupric nitrate 0.001 M at room temperature for the ionic interchange of Cu and Cd. Different times of immersion were performed on the ion exchange solution to obtain different copper concentrations. The immersion time was measured, and the samples were labeled as MXX, being XX the time in seconds of immersion in the Cu ion exchange solution. The objective was to control the band gap value adjusting the doping time. Different samples with the same initial CdS film were dipped for different amount of time. Finally, the annealing process was performed at a temperature of 200 °C for 20 min in a nitrogen atmosphere at a pressure of 10 mmHg to improve the crystallinity of the material and to diffuse the Cu ions into the CdS structure.
The morphology and thickness of the deposited films were characterized by scanning electron microscopy (SEM), employing a JEOL JSM-7800F microscope operated at 15 kV. UV–Vis spectroscopy measurements were performed with a Perkin Elmer Lambda 19 Spectrometer, in a wavelength range from 400 to 800 nm. X-ray photoelectron spectroscopy (XPS) was performed with a Perkin Elmer PHI5100 to study the chemical composition on the different samples; the studies were done with a Mg source at 15 kV and 300 W with a pressure of 4 × 10−8 Torr. A Rigaku Ultima III X-ray diffractometer was used for the X-ray diffraction.
3 Results
Scanning electron microscopy measurements were performed on the CdS and Cu-doped CdS thin films to study their surface morphology and thickness. Figure 1 shows the micrographs of the films with different doping times. As shown in the SEM images, the undoped CdS films are composed of nanostructures with amorphous shapes of CdS with sizes around 100 nm. Upon the inclusion of the Cu-doping, it can be seen that the nanostructure’s shapes remained similar with a slight size decrease of up to 15 nm compared with the undoped material. In addition, the SEM cross-section micrograph shows that the thin film thickness is around 100 nm. This value was not significantly modified by the Cu-doping inclusion.
Figure 2 shows the optical absorption spectra of the Cu-doped CdS and undoped films. The absorbance measurements were performed in a range of wavelengths from 400 to 700 nm. It can be seen that from 600 to 700 nm, the spectra present an almost flat absorption region. The undoped CdS sample (M00) present an increase of absorption at around 500 nm. As the doping time (and hence the doping concentration) increases, the absorbance edge shifts up to 575 nm approximately. This effect was visible to the naked eye since the samples changed its color from a cadmium yellow to a reddish yellow as the doping time increased.
Tauc’s plot method [45] was employed to obtain the optical band gap values of the thin films deposited on a glass substrate. To this end, the equation:
is used, where α is the absorption coefficient, B is a constant, hv is the photon energy, Eg is the band gap and n equals 1/2 for a direct allowed transition [46]. The optical band gap is determined from the parameters obtained of a linear fit of the term (αhv)2 (calculated from the absorption spectrum), whose intersection with the x-axis (in α = 0) provides the optical band gap. This graphic interpretation starts by transforming the wavelength values to energy level using the Eq. (1), which comes from:
To obtain the absorption coefficient from the measured absorbance, it was necessary to assume that all the samples had a thickness of 100 nm which was corroborated from a cross-section SEM micrograph of the samples. To calculate α the following equation was used:
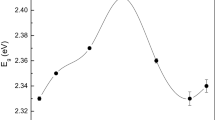
where A is the experimental absorbance and t is the thickness of the sample (100 nm). Figure 3 shows the calculations for the band gap value using the Tauc’s plot method.
As shown in Fig. 3, it is observed that the pure CdS film has a band gap of 2.40 eV. In addition, it was observed that as the doping concentration increases, it produces an enhancement in the band gap reaching a value up to 2.46 eV for the 18 s treated sample, while higher Cu-doping concentration seems to produce a decrease of the band gap. This last effect was previously observed in the Cu-doped CdS material and it was attributed to the effect of the band gap of the CuS, which is 2.16 eV [43].
Figure 4a shows the XPS measurements performed on three samples with different Cu-doping concentrations: (i) an undoped CdS thin film (M00), (ii) a sample with a low doping concentration (M06) and (iii) one with a high doping concentration (M60). As can be seen in Fig. 4a, the three samples exhibit the typical photoelectronic peaks of CdS thin films [47], while the copper ion signals were found only in the Cu-doped thin films as expected. The observed signals correspond to possible CuS compounds with a 2p doublet (2p1/2 and 2p3/2) with binding energies around 952 eV and 932 eV, respectively [48] and the Auger line L3M45M45 (335 eV) associated with copper when a Mg source is utilized for XPS characterization [48] was also found in the doped samples. High-resolution XPS spectra were collected from both the undoped and Cu-doped CdS thin films. Figure 4b shows the characteristic peaks for the undoped CdS 3d doublet at 411.66 eV and 404.92 eV for the Cd 3d3/2 and Cd 3d5/2 respectively, while in the case of the Cu-doped film (Fig. 4c), it can be seen that the incorporation of the copper ions through ion exchange, introduced two small signals at around and 404.5 eV and 411.3 eV, these new signals are thought to be produced by the metallic Cd ions that were replaced in the ion exchange and/or the CdO produced during the reaction. In addition, analyzing the Cu 2p doublet signal from Fig. 4d and e, it can be seen that most of the copper ions were successfully exchanged with the cadmium ions producing CuS. However, a small signal possibly composed by unreacted metallic copper and/or copper oxides are still present. In addition, from the XPS measurements, it was found that the M06 sample, presented a composition of 8% copper, 38% cadmium and 54% sulfur, while the M60 sample had a composition of 17% copper, 34% cadmium, and 49% sulfur. This information confirms that the ion exchange method employed in the present study, produce the replacement of cadmium ions by copper ions, preserving the nearly stoichiometric composition.
The X-ray diffractograms of the undoped CdS and Cu-doped CdS thin films are shown Fig. 5. The results exhibit the preferential cubic CdS \(\left( {1 1 1} \right)\) growth plane at the angular position \(2\theta = 26.75^\circ\) with small contributions of the cubic \(\left( {2 0 2} \right)\) and \(\left( {3 1 1} \right)\) growth planes at \(2\theta = 44.28^\circ\) and \(2\theta = 52.41^\circ\) respectively, which correspond to the zinc-blende structure of CdS indexed in the crystallography open database [49] entry COD-1011251. This crystallographic structure is typically found in CdS thin films obtained by CBD [30, 50]. As the doping concentration increases, the growth of the planes \(\left( {100} \right)\) and \(\left( {101} \right)\) of hexagonal CdS (COD-1011054) located around \(2\theta = 25.35^\circ\) and \(2\theta = 28.52^\circ\) respectively, becomes more evident, suggesting a possible coexistence of the two different crystal structures. Additionally, the grown of the \(\left( {0 1 8} \right)\) plane of the hexagonal phase of CuS (COD-9000062) located around the angular position \(2\theta = 52.51^\circ\) could help to confirm the ion exchange from Cd2+ to Cu2+ with the possible formation of a small population of CuS crystals that could explain the partial growth of the hexagonal wurtzite structure in the CdS matrix of the doped thin films. However, this last signal may be superimposed by the cubic CdS (3 1 1) signal. Scherrer Equation was employed to calculate the crystallite size of the samples before and after the doping process, however, the calculated values were found to be around 13.4 nm with almost negligible variations (within the standard deviation) upon the inclusion of the Cu-doping ions.
A pseudo-MOSFET (prototype) structure was fabricated employing both the undoped and the Cu-doped CdS thin film to investigate its possible application as a field effect transistor. The electrical behavior of the undoped and Cu-doped CdS thin films in a pseudo-MOSFET structure (as shown in Fig. 6a) was analyzed by current vs voltage measurements at room temperature under dark conditions. Figures 6b, c show the IDS–VDS output characteristics measured in the voltage range from 0 to 50 V, for several gate voltages (VGS). As shown in Fig. 6b, it can be observed that the channel current of the “undoped device” does not reach saturation even when 30 volts were applied to the gate contact. In contrast, in Fig. 6c it can be observed that the channel current of the “Cu-doped device” saturate even with 5 volts applied in the gate terminal. This observation could be considered an indication that field effect transistors can be developed by doping CdS thin films, and that they could be controlled with greater channel current precision.
4 Conclusions
Cadmium sulfide thin films were fabricated by the chemical bath deposition technique. The doping process of the CdS thin films was obtained by ion exchange method at room temperature. Analysis of absorbance, energy band gap, XPS, SEM, and XRD were performed to the fabricated thin films to study the effect of the doping concentration on the properties of the material. It was found that a low doping concentration of copper produces an increase in the bandgap value of the CdS thin films, while higher concentrations produces de opposite effect since it could be promoting a greater formation of CuS. Considering the presented results and some previous studies cited here, it can be argued that the copper ions are not just in the surface of the deposited film, but actually they merged into CdS material. This effect is proposed since the thin films shown changes in their band gap values, differences in their crystallinities as well as the elemental composition for the different copper concentrations. In addition, a prototype of a pseudo-MOSFET structure was fabricated employing both the undoped and the Cu-doped CdS thin film to corroborate its possible application as a field effect transistor. However, the copper doping proposed in the present work requires a more precise control in the ion interchange method to obtain smaller and more adjustable copper concentrations. A better control in the doping process will improve the manipulation of the band gap values, producing a more suitable semiconductor for a wider variety of applications since the capability to produce a more reliable semiconductor with a controllable band gap is a priority for semiconductor industry.
References
L. Cheng, Q. Xiang, Y. Liao, H. Zhang, CdS-Based photocatalysts. Energy Environ. Sci. 11(6), 1362–1391 (2018)
P. Rodriguez, N. Muñoz-Aguirre, E. San-Martín Martinez, G. González de la Cruz, S.A. Tomas, O. Zelaya Angel, Synthesis and spectral properties of starch capped CdS nanoparticles in aqueous solution. J. Cryst. Growth 310(1), 160–164 (2008)
M. Muthusamy, S. Muthukumaran, Effect of Cu-doping on structural, optical and photoluminescence properties of CdS thin films. Opt. Int. J. Light Electron. Opt. 126(24), 5200–5206 (2015)
A. AbdolahzadehZiabari, F.E. Ghodsi, Influence of Cu doping and post-heat treatment on the microstructure, optical properties and photoluminescence features of sol-gel derived nanostructured CdS thin films. J. Lumin. 141, 121–129 (2013)
P. Kumar, N. Saxena, A. Agarwal, V. Gupta, Influence of Ag doping concentration on structural and optical properties of CdS thin film. AIP Conf. Proc. 1661, 080017 (2015)
T. Gaewdang, N. Wongcharoen, T. Wongcharoen, Characterisation of CdS/CdTe heterojunction solar cells by current-voltage measurements at various temperatures under illumination. Energy Procedia 15(2011), 299–304 (2012)
R. Ochoa-Landín, J. Sastre-Hernández, O. Vigil-Galan, R. Ramírez-Bon, Chemically deposited CdS by an ammonia-free process for solar cells window layers. Sol. Energy 84(2), 208–214 (2010)
G. Arreola-Jardón, L.A. González, L.A. García-Cerda, B. Gnade, M.A. Quevedo-López, R. Ramírez-Bon, Ammonia-free chemically deposited CdS films as active layers in thin film transistors. Thin Solid Films 519(1), 517–520 (2010)
B. Mereu et al., Field-effect transistor based on nanometric thin CdS films. Mater. Sci. Eng. B 109(1–3), 260–263 (2004)
S.A. Mirsagatov, R.R. Kabulov, M.A. Makhmudov, Injection photodiode based on an n-CdS/p-CdTe heterostructure. Semiconductors 47(6), 825–830 (2013)
T.T. Xuan, J.Q. Liu, R.J. Xie, H.L. Li, Z. Sun, Microwave-assisted synthesis of CdS/ZnS: Cu quantum dots for white light-emitting diodes with high color rendition. Chem. Mater. 27(4), 1187–1193 (2015)
T. Abe, J. Sato, S. Ohashi, M. Watanabe, Y. Kashiwaba, Light emission of a CdS(Cu)/CdS thin-film diode. Phys. Status Solidi Basic Res. 229(2), 1015–1018 (2002)
S.R. Ferrá-González et al., Optical and structural properties of CdS thin films grown by chemical bath deposition doped with Ag by ion exchange. Opt. Int. J. Light Electron. Opt. 125(4), 1533–1536 (2014)
A. Abdolahzadeh Ziabari, F.E. Ghodsi, Growth, characterization and studying of sol-gel derived CdS nanoscrystalline thin films incorporated in polyethyleneglycol: effects of post-heat treatment. Sol. Energy Mater. Sol. Cells 105, 249–262 (2012)
O. Vigil-Galán et al., Physical properties of Bi doped CdTe thin films grown by CSVT and their influence on the CdS/CdTe solar cells PV-properties. Thin Solid Films 515(15 SPEC. ISS.), 5819–5823 (2007)
E.A. Martín-Tovar, R. Castro-Rodríguez, A. Iribarren, Isoelectronic CdTe-doped ZnO thin films grown by PLD. Mater. Lett. 139, 352–354 (2015)
E. Hernández-Rodríguez, M. Loeza-Poot, I. Riech, V. Rejón, J.L. Peña, A comparative study of CdS: F and CdS: O thin films deposited by reactive RF-sputtering technique for window layer application in solar cells. J. Phys. D 48(25), 255102 (2015)
P. Gogoi, Thermally deposited Ag-doped CdS thin film transistors with high-k rare-earth oxide Nd2O3 as gate dielectric. Semiconductors 47(3), 341–344 (2013)
N.A. Shah et al., Physical properties and characterization of Ag doped CdS thin films. J. Alloys Compd. 512(1), 27–32 (2012)
S. Yilmaz, The investigation of spray pyrolysis grown CdS thin films doped with flourine atoms. Appl. Surf. Sci. 357, 873–879 (2015)
R. Panda et al., Carrier recombination in Cu doped CdS thin films: photocurrent and optical studies. Appl. Surf. Sci. 258(12), 5086–5093 (2012)
A. Jaber, S.N. Alamri, M.S. Aida, Ammonia free growth of CdS thin films by chemical bath technique. AIP Conf. Proc. 1370, 81–88 (2011)
J.I. Contreras-Rascón, J. Díaz-Reyes, J.E. Flores-Mena, M. Galvan-Arellano, L.A. Juárez-Morán, R.S. Castillo-Ojeda, Characterization of CBD-CdSe1-ySy deposited at low-temperature for photovoltaic applications. Curr. Appl. Phys. 15(12), 1568–1575 (2015)
P.O. Brien, J. Mcaleese, bath deposition of ZnS and CdS. Technology 8(11), 2309–2314 (1998)
J. Hernández-Borja, Y.V. Vorobiev, R. Ramírez-Bon, Thin film solar cells of CdS/PbS chemically deposited by an ammonia-free process. Sol. Energy Mater. Sol. Cells. 95, 1882–1888 (2011)
M.G. Sandoval-Paz, R. Ramírez-Bon, Analysis of the early growth mechanisms during the chemical deposition of CdS thin films by spectroscopic ellipsometry. Thin Solid Films 517, 6747–6752 (2009)
M.G. Sandoval-Paz, M. Sotelo-Lerma, A. Mendoza-Galvan, R. Ramírez-Bon, Optical properties and layer microstructure of CdS films obtained from an ammonia-free chemical bath deposition process. Thin Solid Films 515, 3356–3362 (2007)
C.D. Lokhande, E.H. Lee, K.D. Jung, O.S. Joo, Ammonia-free chemical bath method for deposition of microcrystalline cadmium selenide films. Mater. Chem. Phys. 91, 200–204 (2005)
D.H. Rose, F.S. Hasoon, R.G. Dhere, D.S. Albin, R.M. Ribelin, X.S. Li, Y. Mahathongdy, T.A. Gessert, P. Sheldon, Fabrication procedures and process sensitivities for CdS/CdTe solar cells. Prog. Photovoltaics Res. Appl. 7, 331–340 (1999)
H. Khallaf, G. Chai, O. Lupan, L. Chow, S. Park, A. Schulte, Characterization of gallium-doped CdS thin films grown by chemical bath deposition. Appl. Surf. Sci. 255, 4129–4134 (2009)
K. Ravichandran, V. Senthamilselvi, Effect of indium doping level on certain physical properties of CdS films deposited using an improved SILAR technique. Appl. Surf. Sci. 270, 439–444 (2013)
J.H. Lee, J.S. Yi, K.J. Yang, J.H. Park, R.D. Oh, Electrical and optical properties of boron doped CdS thin films prepared by chemical bath deposition. Thin Solid Films 431–432, 344–348 (2003)
A.K. Gupta, R. Kripal, EPR and photoluminescence properties of Mn2+ doped CdS nanoparticles synthesized via co-precipitation method. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 96, 626–631 (2012)
A. Jafari, A. Zakaria, Fabrication and modification of chemical deposited nanocrystalline cadmium sulphide thin film in presence of impurity. AIP Conf. Proc. 1482, 617–620 (2012)
S. Mageswari, L. Dhivya, B. Palanivel, R. Murugan, Structural, morphological and optical properties of Na and K dual doped CdS thin film. J. Alloy. Compd. 545, 41–45 (2012)
V.S. Taur, R.A. Joshi, R. Sharma, Annealing-induced modifications in physicochemical and optoelectronic properties of Ag-doped nanostructured CdS thin films. Int. J. Photoenergy 2012, 1–7 (2012)
P. Li, C. Zhang, J. Lian, M. Ren, P. Wang, X. Yu, S. Gao, First-principle study of optical properties of Cu-doped CdS. Opt. Commun. 295, 45–52 (2013)
H.H. Afify, I.K. El Zawawi, I.K. Battisha, Photoelectronic properties of (Cu, Fe, Al) incorporated CdS thin films. J. Mater. Sci. Mater. Electron. 10, 497–502 (1999)
P. J. Sebastian, Appl. Phys. Lett. 62(23), 2956–2958 (1993)
I. Dr, M. Luqman (eds.), Ion Exchange Technology I (Springer, Dordrecht, 2012)
J.B. Rivest, P.K. Jain, Cation exchange on the nanoscale: an emerging technique for new material synthesis, device fabrication, and chemical sensing. Chem. Soc. Rev. 42, 89–96 (2013)
A. Flores-Pacheco, J.I. Contreras-Rascón, J. Diaz-Reyes, P.D. Angel-Vicente, J.P. Enríquez, S.J. Castillo, M.E. Álvarez-Ramos, Stimulation of the photoluminescent properties of CBD-CdS thin films achieved by structural modifications resulting from Ag+ doping. Phys. Status Solidi - Rapid Res. Lett. 11, 1700134 (2017)
A. Mukherjee, M. Fu, P. Mitra, Influence of Zn incorporation in CdS: structural and morphological studies. J. Phys. Chem. Solids 82, 50–55 (2015)
M. Ristova, M. Ristov, XPS profile analysis on CdS thin film modified with Ag by an ion exchange. Appl. Surf. Sci. 181(1–2), 68–77 (2001)
J. Tauc, R. Grigorovici, A. Vancu, Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi (b) 15(2), 627–637 (1966)
M. Muthusamy, S. Muthukumaran, Effect of Cu-doping on structural, optical and photoluminescence properties of CdS thin films. Optik 126(24), 5200–5206 (2015)
M. Stoev, A. Katerski, XPS and XRD study of photoconductive CdS films obtained by a chemical bath deposition process. J. Mater. Chem. 6, 377 (1996)
J.F. Moulder, W.F. Stickle, P.E. Sobol, K.D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, 2nd edn. Edein Prairie, MN: Perkin-Elmer Corporation Physical Electronics Division (1992)
S. Gražulis, D. Chateigner, R.T. Downs, A.F.T. Yokochi, M. Quirós, L. Lutterotti, E. Manakova, J. Butkus, P. Moeck, A. Le Bail, Crystallography Open Database – an open-access collection of crystal structures. J. Appl. Crystallogr. 42(4), 726–729 (2009)
H. Moualkia, S. Hariech, M.S. Aida, Structural and optical properties of CdS thin films grown by chemical bath deposition. Thin Solid Films 518(4), 1259–1262 (2009)
Acknowledgements
The author acknowledges the CONACyT projects: FORDECYT 272894, Basic Science 242508 and National Laboratories 294452 for the support. The authors express our thanks to the nanoFAB Laboratory for the use of its facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diaz-Grijalva, O.I., Berman-Mendoza, D., Flores-Pacheco, A. et al. Cu-doped CdS thin films by chemical bath deposition and ion exchange. J Mater Sci: Mater Electron 31, 1722–1730 (2020). https://doi.org/10.1007/s10854-019-02690-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02690-2