Abstract
Hierarchical MnO2@NiCo2O4 core–shell nanostructures are well fabricated via a simple two-step hydrothermal process. The MnO2@NiCo2O4 core–shell nanostructures materials electrode presents a high capacitance of 684 F g−1 at 2 A g−1 current density, 40 times higher than that of the single MnO2 nanowires electrode. And 87.4% retain is approached even at a high current density of 15 A g−1, showing satisfactory rate capability. Furthermore, the theoretical analysis reveals the surface capacitance contribution is predominant in the capacitive contribution. The asymmetric supercapacitor assembled with MnO2@NiCo2O4 exhibited a maximum energy density of 35.6 Wh kg−1 and a maximum power density of 745.1 W kg−1. After 7000 charge–discharge cycling at a current density of 4 A g−1, it still can maintain 90% of the initial capacitance. These results suggest that MnO2@NiCo2O4 is the promising candidate of supercapacitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to the intensification of environmental pollution and the continuous depletion of fuel energy, it has become an urgent problem to develop green energy storage device to meet the needs of social development [1,2,3]. Supercapacitors have safe operating voltages and excellent electrochemical performance [4, 5]. There are three kinds of supercapacitor electrodes, which are carbon-based materials [6,7,8], metal oxide materials [9,10,11] and conductive polymer materials (polyaniline, polypyrrole, etc.) [12, 13]. Different from carbon-based and conductive polymer electrode materials, transition metal oxides have unique physical and chemical properties, as well as the abundant reserves, low cost, good thermal stability and green pollution-free characteristics. Most of the transition metal oxides have been investigated as electrochemical energy storage owing to the higher capacitance and energy density [14,15,16,17,18,19], including cobalt trioxide, nickel oxide, manganese oxide and nickel cobalt oxide (NiCo2O4) [20,21,22,23], etc.
Among them, MnO2 is easy to synthesize by various synthetic means such as hydrothermal method, electrodeposition, chemical vapor deposition and the like [24,25,26]. Moreover, it has a variety of morphologies, such as nanowires, nanotubes and nanosheets. In terms of performance, MnO2 nanomaterials have good electrical conductivity and outstanding cycle stability. For example, Nam et al. synthesized nano-flowered and nano-wired MnO2 by ultrasonic processing with a specific capacitance of 300 F g−1 at a scan rate of 5 mV s−1 [27]. Yang et al. prepared amorphous MnO2 using potassium permanganate and trihydroxyethylamine, and it showed a specific capacitance of 251 F g−1 at 2 mv s−1 scanning rate [28]. However, the capacitance of pure MnO2 electrode is generally low and it is difficult to achieve breakthroughs in electrochemical performance. In recent years, some researchers have studied the core–shell nanocomposites with MnO2 as the core and other transition metal oxides as the shell [29,30,31], which exhibiting good supercapacitor performance. Among the transition metal oxides, NiCo2O4 has the high theoretical capacitance, environmental friendliness and low cost [32,33,34]. Combining with the nanostructured NiCo2O4 will exhibit excellent electrochemical performance in energy storage equipment. For example, the MnO2@NiCo2O4 core/shell nanospheres and NiCo2O4/MnO2 nanosheet arrays have presented good electrochemical performance in supercapacitors [35, 36].
In this paper, the one-dimensional MnO2@NiCo2O4 core–shell nanowires were successfully synthesized by a simple two-step hydrothermal method. As a supercapacitor electrode, the specific capacitance was 684 F g−1 at 2 A g−1 current density, and 87.4% remain at higher current density of 15 A g−1, exhibiting good rate performance. In addition, MnO2@NiCo2O4, porous carbon and KOH solution were, respectively, used as the positive electrode, negative electrode and electrolyte to assemble the asymmetric supercapacitor. The device exhibited a specific capacitance of 114 F g−1 at a current density of 1 A g−1. The capacitance still remains 90% after 7000 cycles at the current density of 4 A g−1. The results show that the one-dimensional MnO2@NiCo2O4 core–shell nanowires are promising candidate for excellent supercapacitor material.
2 Experimental section
2.1 Preparation of MnO2 nanowires
This is a traditional hydrothermal synthesis process. Ten milliliters of KMnO4 aqueous solution (0.04 mol/L) and 10 ml of NH4Cl aqueous solution (0.04 mol/L) were placed in a beaker and magnetically stirred for 30 min. Next, the mixture was poured in 50-ml Teflon-lined autoclave, sealed and kept at 180 oC for 20 h. After the hydrothermal reaction, the product was washed with deionized water and absolute ethanol, vacuum dried at 60 oC for 12 h to obtain the MnO2 nanowires.
2.2 Synthesis of one-dimensional MnO2@NiCo2O4 core–shell nanowires
Specifically, 0.2 mmol of Ni(NO3)2∙6H2O, 0.4 mmol of Co(NO3)2∙6H2O and 4 mmol of C6H12N4 were dissolved in 30 mL of an aqueous ethanol solution (ethanol: deionized water = 1:1) and magnetically stirred for 30 min to form a pink solution. Then, 0.4 mmol of MnO2 nanowires was put in the above solution and sonicated for 8 min. Then, the mixture was poured into a 50-ml polytetrafluoroethylene stainless steel autoclave, sealed and heated at 90 °C for 12 h. After the completion of the reaction, the product washed for three times with deionized water and absolute ethanol, dried at 60 oC for 12 h to obtain one-dimensional MnO2@NiCo2O4 core–shell nanowires.
2.3 Materials characterization
The morphology of the material was investigated by a scanning electron microscope (FESEM, Hitachi-S4800) at an accelerating voltage of 5 kV. The structure of the material was obtained using a transmission electron microscope (TEM, Hitachi-HT7700) at an accelerating voltage of 100 kV and a high-resolution transmission electron microscope (HRTEM, FEI Tacnai G2) at an acceleration voltage of 200 kV. The crystal structure was obtained by X-ray powder diffraction (XRD) by collecting the patterns using a diffractometer (Bruker D8) equipped with a CuKα radiation source (λ = 1.54060 Å).
2.4 Electrochemical measurements
Electrochemical studies were performed via a CHI 660E electrochemical workstation in 3 M aqueous KOH solution. The process was performed in the three-electrode cell system: the counter electrode of platinum wire, the reference electrode of saturated calomel electrode and the working electrode fabricated by mixing the active material (sample, weight 80%), conductive material (acetylene black, weight 10%) and binder (polytetrafluoroethylene, 10% by weight). The mass loading of the active material was calculated to be about 2.96 mg cm−2.
3 Results and discussion
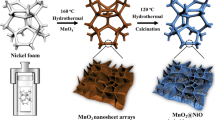
In Scheme 1, the fabrication of hierarchical MnO2@NiCo2O4 core–shell nanostructures was schematically depicted, involving a simple two-step hydrothermal synthesis process. Firstly, 10 ml of KMnO4 and 10 ml of NH4Cl were hydrothermally reacted to form brown MnO2 nanowires monomers directly (as shown in Scheme 1b). Subsequently, through the second hydrothermal process, NiCo2O4 nanosheets were grown on the MnO2 nanowires to construct hierarchical one-dimensional MnO2@NiCo2O4 core–shell nanowires (as shown in Scheme 1c).
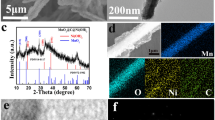
Figure 1a shows the diffraction peaks of the MnO2 nanowires and MnO2@NiCo2O4 core–shell nanowires. The peaks located at 2 θ = 12.7, 18.1, 28.7, 37.6 and 50° are indexed to the (110), (200), (310), (121) and (411) planes of MnO2 (JCPDS File No. 72-1982), respectively. At the same time, the peaks at 18.9, 31.0, 36.6 and 44.5o correspond to the (111), (220), (311) and (400) planes of NiCo2O4. The results reveal that the chemical compositions of the product are MnO2 and NiCo2O4.
XRD patterns of MnO2 and MnO2@NiCo2O4 core–shell nanowires; b low-magnification SEM image of MnO2@NiCo2O4; c high-magnification SEM morphology of MnO2@NiCo2O4; d the low-magnification TEM image of MnO2@NiCo2O4; e the high-magnification TEM image of MnO2@NiCo2O4; and f the HRTEM image of MnO2@NiCo2O4
The SEM and TEM have been performed to study the microstructure of the products. Figure 1b displays the low-magnification SEM image of MnO2@NiCo2O4, showing that the MnO2@NiCo2O4 nanomaterial consists of one-dimensional nanowires with uniform distribution with the average diameter of 130 nm. And the nanosheets are evenly anchored on the surface of the nanowires via the high-magnification SEM image (Fig. 1c). It is also clearly seen that the nanowires are uniformly covered by the nanosheets to form a layered core–shell nanostructure through the low-magnification TEM morphology (Fig. 1d). And it can be observed that the nanoflakes are composed of very small crystal grains in the high-magnification TEM morphology (Fig. 1e). Figure 1f shows the high-resolution TEM (HRTEM) image of MnO2@NiCo2O4. The clear interplanar spacings of 0.310 nm and 0.242 nm are consistent with the (310) plane of MnO2 and the (311) plane of NiCo2O4, respectively. Based on the above results, we believe that the layered one-dimensional MnO2@NiCo2O4 nanowires have been successfully synthesized.
The cyclic voltammetry (CV) curves of the MnO2 electrode are measured at various scan rates ranging from 5 mV s−1 to 80 mV s−1, as shown in Fig. 2a. There is a distinct redox peak in the CV curve, and no obvious distortion at the high scanning rate of 80 mV s−1, exhibiting clearly the pseudocapacitor capacitance property. The GCD curves of the MnO2 electrode ranging from 2 to 10 A g−1 are investigated, as shown in Fig. 2b. The specific capacitance (Cm (F g−1)) of the electrode based on the mass of the active materials is calculated according to the following equations:
CV curves of the MnO2 electrode at various scan rates; b galvanostatic charging and discharging curves of the MnO2 electrode at a series of current densities; c CV curves of the MnO2@NiCo2O4 electrode at various scan rates; d galvanostatic charge–discharge curves of the MnO2@NiCo2O4 electrode at a series of current densities; the specific capacitance as a function of the current densities of e MnO2 electrode and f MnO2@NiCo2O4 electrode
where I, ∆t, ∆V and m are the current (A) during the discharge process, discharge time(s), potential window (V) and mass of the active materials (g), respectively. After calculation, the specific capacitance of the one-dimensional MnO2 nanowires at current densities of 2, 4, 6, 8 and 10 A g−1 are 17.7, 14, 12, 10.1 and 8.89 F g−1, respectively, as shown in Fig. 2e. Figure S1 shows the comparative CV curves of MnO2@NiCo2O4, acetylene black and nickel foam at a scan rate of 0.8 mV s−1 in the potential range from 0 to 0.5 V. Obviously, the area of the CV curve enclosed by acetylene black or nickel foam is much smaller than that of the MnO2@NiCo2O4 electrode, indicating that the proportion of capacitance contributed from acetylene black and nickel foam in MnO2@Nio2O4 system can be neglected. It is easy to see that the specific capacitance of the MnO2 nanowires is low and has small rate capability of 50.2%.
Compared with one-dimensional MnO2 nanowires, MnO2@NiCo2O4 has a significant performance improvement (Fig. 2c). With increasing the scan rate, the peaks of the anode and cathode move toward more negative and positive potentials, respectively, suggesting a quasi-reversible characteristic of the redox reaction. Even at the scan rate of 80 mV s−1, the CV curve has no obvious distortion, indicating the ideal CV stability of the MnO2@NiCo2O4. Figure 2d shows the GCD performance of the MnO2@NiCo2O4 electrode at various current densities from 2 to 8 A g−1. Clearly, the curves under low current density appear a platform, which represents the redox reactions [37]. The calculated specific capacitances are 684, 665, 660, 640 and 600 F g−1 at the current densities of 2, 4, 6, 10 and 15 A g−1, respectively. It is worth noting that the MnO2@NiCo2O4 electrode also shows a high specific capacitance of 600 F g−1 when the current density is up to 15 A g−1, exhibiting the excellent rate capacity. In addition, compared to a single MnO2, the specific capacitance of the core–shell electrode at 2 A g−1 is almost 40 times higher. From the above analysis, it can be stated that the core–shell structure can greatly enhance the electrochemical properties of the material.
For electrode materials, cycling stability is also a very important parameter [38]. After 4000 cycles at a current density of 6 A g−1, the MnO2@NiCo2O4 electrode is capable of retaining 85.6% of the initial specific capacitance, indicating that electrode material has good electrochemical stability, as shown in Fig. 3a. In the first 800 cycles, the capacitance of the electrode material gradually increases to 850 F g−1, and the reason could be the activation of the electrode material. Then the capacitance begins to drop slowly. However, after 4000 cycles, it still can retain a capacitance of 85.6%. In order to verify whether the MnO2@NiCo2O4 electrode morphology transforms after 4000 cycles, the SEM test after cycling was further investigated, as shown in Fig. 3b. By comparison, after 4000 cycles, the core–shell structure still exists and the surface of the MnO2@NiCo2O4 electrode is considerably rougher. The layered core–shell nanostructures keep stability except for a small amount of collapse during the repeated charging/discharging process, implying that the unique nanostructures can effectively alleviate the volume expansion. First, the 1 dimensional MnO2 nanowire core provide a steady structural support during charge–discharge. Second, the interconnected NiCo2O4 nanosheets provide more void space, which buffering the volume change during charge/discharge [39,40,41].
Cycling stability of MnO2@NiCo2O4 electrode at a current density of 6 A g−1, insets: the first and last 7 cycling GCD curves; b the SEM image of MnO2@NiCo2O4 electrode after 4000 cycling; c EIS of the electrode at the 1st and 100th cycling, insets: the high frequency; d the phase angles for impedance curves of the 1st and 100th cycling
Electrochemical impedance spectra (EIS) of the MnO2@NiCo2O4 electrode before and after 100 cycles were investigated, as shown in Fig. 3c. All Nyquist diagrams consist of two parts, a depressed semicircle in the high-frequency region representing the charge transfer resistance, a slope line in the low-frequency region representing the Warburg impedance attributed to the diffusion resistance of the electrolyte [42]. In the low-frequency region, the MnO2@NiCo2O4 core–shell nanowire exhibits low Warburg impedance, indicating the facilitated electrolyte diffusion to the surface of the electrodes. The real axis intercept in the high-frequency region represents the volume resistance of the electrochemical system. There was only a slight difference between the 1st and 100th cycling, suggesting the good ion diffusion and conduction kinetics.
Furthermore, the Bode plot of the 1st and 100th cycling also shown in Fig. 3d. It can be found that the operating frequencies of the 1st and 100th cycling are 0.60 and 0.53 Hz, and the corresponding characteristic relaxation time constants (τ0) are 1.67 and 1.88 s, respectively. The difference between before and after the cycle is small, revealing that the electrode has stable electrolyte permeation and ion diffusion kinetics. Further, it can be seen that this material has better electrochemical properties than the other MnO2-based electrode materials [31, 43,44,45,46,47], as given in Table 1.
In order to further understand the superior electrochemical performance of the MnO2@NiCo2O4 core–shell nanowires, we performed a kinetic analysis of the electrochemical. Figure 4a displays the CVs of the MnO2@NiCo2O4 electrode from 0.2 to 0.8 mV s−1. It is known that charge-storage involves two types of mechanisms: the non-faradaic process from the electric double-layer (EDLCs) and the faradaic contribution produced by a redox reaction. The latter can be divided into the diffusion-controlled process and the surface pseudocapacitive contribution arising from the charge transfer at or the near surface [48].
CV curves at different scan rates of the MnO2@NiCo2O4 electrode; b relationship between logarithm peak current versus logarithm scan rates; c capacitive contribution (wine for MnO2, blue for NiCo2O4 and red for MnO2@NiCo2O4) and diffusion contribution (gray) at 0.8 mV s−1; d the percentage of capacitance contribution at different scan rates
The current (i) and scan rate (v) obey following equation [49]:\( i = av^{b} \), where a is constants, and the value of b is between 0.5 ~ 1. For a diffusion-controlled process, the b value is close to 0.5, while for surface pseudocapacitive contribution, the value of b approach 1. In Fig. 4b, the slope of log i versus log v represents the b value. The b value (0.72) of the MnO2@NiCo2O4 electrode is higher the MnO2 and NiCo2O4, suggesting that the surface capacitance control capacitance kinetics of MnO2@NiCo2O4 electrode dominates.
To quantify the contribution from diffusion-controlled process and surface capacitive effect, we can separate the current response (i) at a specific potential (V) into a diffusion control reaction and a capacitive effect. According to the equation [50, 51]:
By drawing i(v)/v1/2 versus v1/2 at multiple potentials, we can get the values of k1 (slope) and k2 (intercept) from the straight line to distinguish the ratio of capacitance contribution under different charge-storage mechanisms. The plot of i(v)/v1/2 versus v1/2 has been presented, as shown in Fig. S2. In order to demonstrate the capacitive contribution, the data are obtained at small sweep speeds from 0.2 to 0.8 mV s−1. From Fig. 4c, there is a significant fraction of surface capacitive (86%) in the MnO2@NiCo2O4 core–shell nanowires electrode, much higher than 35% of the MnO2 sample and 24% of the NiCo2O4 sample at the 0.8 mV s−1. This result is in good agreement with its higher b value, suggesting the excellent kinetics of the MnO2@NiCo2O4 core–shell nanowires electrode. The capacitance contribution of the MnO2@NiCo2O4 electrode is 69%, 77%, 82% at the scan rate of 0.2, 0.4 and 0.6 mV s−1 (Fig. 5), respectively, indicating the capacitance-dominated capacitance is leading in the total capacitance. As the sweep speed increases, the surface capacitance contribution gradually increases throughout the current response (Fig. 4d), which is consistent with the expected results.
Capacitance contribution to charge storage (wine for MnO2, blue for NiCo2O4 and red for MnO2@NiCo2O4) and diffusion contribution (gray) of different electrode at different scan rates: a 0.2, d 0.4 and g 0.6 mV s−1 of the MnO2; b 0.2, e 0.4 and h 0.6 mV s−1 of the NiCo2O4; c 0.2, f 0.4 and i 0.6 mV s−1 of the MnO2@NiCo2O4
It is well known that diffusion control behavior occurs primarily in bulk materials or in large dimensions. Therefore, these materials generally exhibit slow kinetic characteristics in electrochemical performance [52]. However, when the material is designed in the nanometer dimension, its electrochemical performance can be significantly improved. For example, LiCoO2 from bulk to nanoscale exhibits significant surface capacitance control [53]. For the MnO2@NiCo2O4 core–shell nanowires, as the outer shell, the nano-sized NiCo2O4 exposes more active sites, which can more fully contact the electrolyte, shorten the ion transmission distance and accelerate the transmission speed, resulting in a high level of capacitive energy storage.
An asymmetric supercapacitor (ASC) using MnO2@NiCo2O4 as positive electrode, porous carbon as negative electrode in aqueous 3 M KOH was fabricated. The potential windows of MnO2@NiCo2O4 and porous carbon are from 0 to 0.5 V and −1.0 to 0 V (Fig. 6a), respectively, indicating the assembled supercapacitor can provide an operating potential of 0–1.50 V in 3 M KOH. As expected, the big operating voltage of the ASC device is stable at 1.5 V, as shown in Fig. 6b. Figure 6c shows the GCD curves of the MnO2@NiCo2O4//C, and the specific capacitances are 114, 104, 94, 85 and 77 F g−1 at current densities of 1, 2, 3, 4 and 5 A g−1, respectively. The rate performance of the MnO2@NiCo2O4//C asymmetric supercapacitor device is shown in Fig. 6d. The MnO2@NiCo2O4//C device can maintain 67.5% of capacitance retention at a high current density of 5 A g−1, exhibiting the better rate capability.
CV curves of MnO2@NiCo2O4 and porous carbon at a scan rate of 60 mV s−1; b CV curves of the MnO2@NiCo2O4//C ASC at various scan rates; c GCD curves of the MnO2@NiCo2O4//C device at various current densities; d the rate performance of the ASC; e Ragone plots of theMnO2@NiCo2O4 device; f cycling performances of the MnO2@NiCo2O4//C device, inset: the 1st and last 7 GCD cycles
Energy density and power density are critical indicators for the practical application of supercapacitors. Ragone plots (energy density vs power density) have been shown in Fig. 6e. The power density and energy density of the ASC were calculated using the following equations:
Where E is the energy density, C represents the specific capacitance of the ASC, ΔV refers to the operating voltage of the device, Δt is the discharge time, and P is the power density. The MnO2@NiCo2O4//C asymmetric supercapacitor exhibits a high energy density of 35.6 Wh kg−1 at power density of 745.1 W kg−1. When the current density is increased to 5 A g−1, an energy density can still be maintained at 24.1 Wh kg−1, while the power density can reach 3772.2 W kg−1. It can also be seen from the figure that the curve is relatively stable and there is no sharp drop. Therefore, the MnO2@NiCo2O4//C asymmetric supercapacitor has a potential application in high energy density storage.
The cycling stability test of the MnO2@NiCo2O4//C asymmetric supercapacitor was conducted at a current density of 4 A g−1, as shown in Fig. 6f. The asymmetrical supercapacitor has a stable cycling performance without major fluctuations. After 7000 cycles, the specific capacitance of the ASC retains 90% of initial value. In addition, the 1st and last 7 GCD cycles (the inset of Fig. 6f) have few deformation, suggesting the good cycling performance. All these results demonstrate the good performance of the ASC and the practical application in the energy density storage.
4 Conclusions
In summary, we successfully designed NiCo2O4 nanosheets coated on MnO2 nanowires to assemble one-dimensional MnO2@NiCo2O4 core–shell nanowires by simple two-step hydrothermal method. The electrochemical performance of the core–shell heterostructure is much higher that of MnO2 nanowire monomers. In particular, the specific capacitance of the one-dimensional MnO2@NiCo2O4 electrode is nearly 40 times higher than that of MnO2 at 2 A g−1, attributed to its unique core–shell structure. And the surface capacitance contribution is leading in the capacitive storage via the kinetically analysis. In addition, the asymmetric supercapacitor device exhibits high energy density of 35.6 Wh Kg−1 with a corresponding power density of 745.1 W Kg−1. Therefore, the MnO2@NiCo2O4 core–shell nanowires can be used as potential electrode materials.
References
L.-F. Chen, Y. Feng, H.-W. Liang, Z.-Y. Wu, S.-H. Yu, Three-dimensional carbon nanofiber architectures for electrochemical energy storage devices. Adv Energy Mater 7, 201700826 (2017)
M. Chen, Y. Zhang, L. Xing, Y. Liao, Y. Qiu, S. Yang, W. Li, Morphology-conserved transformations of metal-based precursors to hierarchically porous micro-/nanostructures for electrochemical energy conversion and storage. Adv. Mater. 29, 201607015 (2017)
H. Wang, C. Zhu, D. Chao, Q. Yan, H.J. Fan, Nonaqueous hybrid lithium-ion and sodium-ion capacitors. Adv. Mater. 29, 201702093 (2017)
Y. Chen, K. Cai, C. Liu, H. Song, X. Yang, High-performance and breathable polypyrrole coated air-laid paper for flexible all-solid-state supercapacitors. Adv Energy Mater 7, 201701247 (2017)
W. Liu, M. Ulaganathan, I. Abdelwahab, X. Luo, Z. Chen, S.J.R. Tan, X. Wang, Y. Liu, D. Geng, Y. Bao, J. Chen, K.P. Loh, Two-dimensional polymer synthesized via solid-state polymerization for high-performance supercapacitors. ACS Nano 12, 852–860 (2018)
H. Jia, Y. Cai, X. Zheng, J. Lin, H. Liang, J. Qi, J. Cao, J. Feng, W. Fei, Mesostructured carbon nanotube-on-MnO2 nanosheet composite for high-performance supercapacitors. ACS Appl. Mater. Interfaces. 10, 38963–38969 (2018)
Z. Lu, J. Foroughi, C. Wang, H. Long, G.G. Wallace, Superelastic hybrid CNT/graphene fibers for wearable energy storage. Adv Energy Mater 8, 201702047 (2018)
M. Zou, W. Zhao, H. Wu, H. Zhang, W. Xu, L. Yang, S. Wu, Y. Wang, Y. Chen, L. Xu, A. Cao, Single carbon fibers with a macroscopic-thickness, 3D highly porous carbon nanotube coating. Adv. Mater. 30, 201704419 (2018)
B.Y. Guan, A. Kushima, L. Yu, S. Li, J. Li, X.W. Lou, Coordination polymers derived general synthesis of multishelled mixed metal-oxide particles for hybrid supercapacitors. Adv. Mater. 29, 201605902 (2017)
K. Qiu, M. Lu, Y. Luo, X. Du, Engineering hierarchical nanotrees with CuCo2O4 trunks and NiO branches for high-performance supercapacitors. J Mater Chem A 5, 5820–5828 (2017)
R.R. Salunkhe, Y.V. Kaneti, Y. Yamauchi, Metal-organic framework-derived nanoporous metal oxides toward supercapacitor applications: progress and prospects. ACS Nano 11, 5293–5308 (2017)
K. Zhou, Y. He, Q. Xu, Q.E. Zhang, A.A. Zhou, Z. Lu, L.-K. Yang, Y. Jiang, D. Ge, X.Y. Liu, H. Bai, A Hydrogel of ultrathin pure polyaniline nanofibers: oxidant-templating preparation and supercapacitor application. ACS Nano 12, 5888–5894 (2018)
K. Shu, Y. Chao, S. Chou, C. Wang, T. Zheng, S. Gambhir, G.G. Wallace, “Tandem” strategy to fabricate flexible graphene/polypyrrole nanofiber film using the surfactant-exfoliated graphene for supercapacitors. ACS Appl. Mater. Interfaces. 10, 22031–22041 (2018)
D. Dastan, A. Banpurkar, Solution processable sol-gel derived titania gate dielectric for organic field effect transistors. J. Mater. Sci. - Mater. Electron. 28, 3851–3859 (2017)
D. Dastan, N. Chaure, M. Kartha, Surfactants assisted solvothermal derived titania nanoparticles: synthesis and simulation. J. Mater. Sci. 28, 7784–7796 (2017)
D. Dastan, S.L. Panahi, N.B. Chaure, Characterization of titania thin films grown by dip-coating technique. J. Mater. Sci. 27, 12291–12296 (2016)
X.-T. Yin, W.-D. Zhou, J. Li, P. Lv, Q. Wang, D. Wang, F.-Y. Wu, D. Dastan, H. Garmestani, Z. Shi, S. Talu, Tin dioxide nanoparticles with high sensitivity and selectivity for gas sensors at sub-ppm level of hydrogen gas detection. J. Mater. Sci. 30, 14687–14694 (2019)
X. Zhu, J. Yang, D. Dastan, H. Garmestani, R. Fan, Z. Shi, Fabrication of core-shell structured Ni@BaTiO3 scaffolds for polymer composites with ultrahigh dielectric constant and low loss. Compos. A 125, 105521 (2019)
D. Dastan, P.U. Londhe, N.B. Chaure, Characterization of TiO2 nanoparticles prepared using different surfactants by sol-gel method. J. Mater. Sci. 25, 3473–3479 (2014)
J. Hao, S. Peng, H. Li, S. Dang, T. Qin, Y. Wen, J. Huang, F. Ma, D. Gao, F. Li, G. Cao, A low crystallinity oxygen-vacancy-rich Co3O4 cathode for high-performance flexible asymmetric supercapacitors. J Mater Chem A 6, 16094–16100 (2018)
Z. Wang, F. Wei, Y. Sui, J. Qi, Y. He, Q. Meng, A novel core-shell polyhedron Co3O4/MnCo2O4.5 as electrode materials for supercapacitors. Ceram. Int. 45, 12558–12562 (2019)
P. Balasubramanian, M. Annalakshmi, S.-M. Chen, T. Sathesh, T.-K. Peng, T. Balamurugan, Facile solvothermal preparation of Mn2CuO4 microspheres: excellent electrocatalyst for real-time detection of H2O2 released from live cells. ACS Appl. Mater. Interfaces. 10, 43543–43551 (2018)
X.L. Guo, M. Kuang, F. Dong, Y.X. Zhang, Monodispersed plum candy-like MnO2 nanosheets-decorated NiO nanostructures for supercapacitors. Ceram. Int. 42, 7787–7792 (2016)
S. Zhu, L. Li, J. Liu, H. Wang, T. Wang, Y. Zhang, L. Zhang, R.S. Ruoff, F. Dong, Structural directed growth of ultrathin parallel birnessite on beta-MnO2 for high-performance asymmetric supercapacitors. ACS Nano 12, 1033–1042 (2018)
X. Meng, L. Lu, C. Sun, Green synthesis of three-dimensional MnO2/graphene hydrogel composites as a high-performance electrode material for supercapacitors. ACS Appl. Mater. Interfaces. 10, 16474–16481 (2018)
Qi Gao, Jinxing Wang, Bin Ke, Jingfeng Wang, Yanqiong Li, Fe doped δ-MnO2 anoneedles as advanced supercapacitor electrodes. Ceram. Int. 44, 18770–18775 (2018)
H.-S. Nam, J.-K. Yoon, J.M. Ko, J.-D. Kim, Electrochemical capacitors of flower-like and nanowire structured MnO2 by a sonochemical method. Mater. Chem. Phys. 123, 331–336 (2010)
Y.-J. Yang, E.-J. Liu, L.-M. Li, Z.-Z. Huang, H.-J. Shen, X.-X. Xiang, J Nanostructured amorphous MnO2 prepared by reaction of KMnO4 with triethanolamine. J Alloys Compd 505, 555–559 (2010)
S. Kim, H.J. Jung, J.C. Kim, K.-S. Lee, S.S. Park, V.P. Dravid, K. He, H.Y. Jeong, In situ observation of resistive switching in an asymmetric graphene oxide bilayer structure. ACS Nano 12, 7335–7342 (2018)
X. Lu, C. Shen, Z. Zhang, E. Barrios, L. Zhai, Core-shell composite fibers for high-performance flexible supercapacitor electrodes. ACS Appl. Mater. Interfaces. 10, 4041–4049 (2018)
X. Wang, H. Xia, J. Gao, B. Shi, Y. Fang, M. Shao, Enhanced cycle performance of ultraflexible asymmetric supercapacitors based on a hierarchical MnO2@NiMoO4 core-shell nanostructure and porous carbon. J Mater Chem A 4, 18181–18187 (2016)
D.S. Sun, Y.H. Li, Z.Y. Wang, X.P. Cheng, S. Jaffer, Y.F. Zhang, Understanding the mechanism of hydrogenated NiCo2O4 nanograss supported on Ni foam for enhanced-performance supercapacitors. J Mater Chem A 4, 5198–5204 (2016)
S. Sun, S. Wang, S. Li, Y. Li, Y. Zhang, J. Chen, Z. Zhang, S. Fang, P. Wang, Asymmetric supercapacitors based on a NiCo2O4/three dimensional graphene composite and three dimensional graphene with high energy density. J Mater Chem A 4, 18646–18653 (2016)
F.-X. Ma, L. Yu, C.-Y. Xu, X.W. Lou, Self-supported formation of hierarchical NiCo2O4 tetragonal microtubes with enhanced electrochemical properties. Energy Environ. Sci. 9, 862–866 (2016)
Y. Zhou, L. Ma, M.Y. Gan, M.H. Ye, X.R. Li, Y.F. Zhai, F.B. Yan, F.F. Cao, Monodisperse MnO2@NiCo2O4 core/shell nanospheres with highly opened structures as electrode materials for good-performance supercapacitors. Appl. Surf. Sci. 444, 1–9 (2018)
S.W. Zhang, B.S. Yin, C. Liu, Z.B. Wang, D.M. Gu, Self-assembling hierarchical NiCo2O4/MnO2 nanosheets and MoO3/PPy core-shell heterostructured nanobelts for supercapacitor. Chem Engin J 312, 296–305 (2017)
X. Wang, B. Shi, Y. Fang, F. Rong, F. Huang, R. Que, M. Shao, High capacitance and rate capability of a Ni3S2@CdS core-shell nanostructure supercapacitor. J Mater Chem A 5, 7165–7172 (2017)
J. Han, Y. Dou, J. Zhao, M. Wei, D.G. Evans, X. Duan, Flexible CoAl LDH@PEDOT core/shell nanoplatelet array for high-performance energy storage. Small 9, 98–106 (2013)
D. Zhao, X. Wu, C. Guo, Hybrid MnO2@NiCo2O4 nanosheets for high performance asymmetric supercapacitors. Inorg Chim Front 5, 1378–1385 (2018)
Z. Ma, G. Shao, Y. Fan, M. Feng, D. Shen, H. Wang, Fabrication of high-performance all-solid-state asymmetric supercapacitors based on stable alpha-MnO2@NiCo2O4 core shell heterostructure and 3D-nanocage N-doped porous carbon. Acs Sustain Chem Eng 5, 4856–4868 (2017)
L. Su, L. Hou, S. Di, J. Zhang, X. Qin, Plumage-like MnO2@NiCo2O4 core-shell architectures for high-efficiency energy storage: the synergistic effect of ultralong MnO2 “scaffold” and ultrathin NiCo2O4 “fluff”. Ionics 24, 3227–3235 (2018)
L. Peng, X. Ji, H. Wan, Y. Ruan, K. Xu, C. Chen, L. Miao, J. Jiang, Nickel sulfide nanoparticles synthesized by microwave-assisted method as promising supercapacitor electrodes: an experimental and computational study. Electrochim. Acta 182, 361–367 (2015)
W. Dang, C. Dong, Z. Zhang, G. Chen, Y. Wang, H. Guan, Self-grown MnO2 nanosheets on carbon fiber paper as high-performance supercapacitors electrodes. Electrochim. Acta 217, 16–23 (2016)
L. Wang, Y. Ouyang, X. Jiao, X. Xia, W. Lei, Q. Hao, Polyaniline-assisted growth of MnO2 ultrathin nanosheets on graphene and porous graphene for asymmetric supercapacitor with enhanced energy density. Chem Engin J 334, 1–9 (2018)
D. Wu, S. Xu, M. Li, C. Zhang, Y. Zhu, Y. Xu, W. Zhang, R. Huang, R. Qi, L. Wang, P.K. Chu, Hybrid MnO2/C nano-composites on a macroporous electrically conductive network for supercapacitor electrodes. J Mater Chem A 3, 16695–16707 (2015)
N. Zhang, C. Fu, D. Liu, Y. Li, H. Zhou, Y. Kuang, Three-dimensional pompon-like MnO2/graphene hydrogel composite for supercapacitor. Electrochim. Acta 210, 804–811 (2016)
S. Zhu, H. Zhang, P. Chen, L.-H. Nie, C.-H. Li, S.-K. Li, Self-assembled three-dimensional hierarchical graphene hybrid hydrogels with ultrathin beta-MnO2 nanobelts for high performance supercapacitors. J Mater Chem A 3, 1540–1548 (2015)
X. Xia, D. Chao, Y. Zhang, J. Zhan, Y. Zhong, X. Wang, Y. Wang, Z.X. Shen, J. Tu, H.J. Fan, Generic synthesis of carbon nanotube branches on metal oxide arrays exhibiting stable high-rate and long-cycle sodium-ion storage. Small 12, 3048–3058 (2016)
H. Lindstrom, S. Sodergren, A. Solbrand, H. Rensmo, J. Hjelm, A. Hagfeldt, S.E. Lindquist, J Phys Chem B 101, 7717–7722 (1997)
J. Wang, J. Polleux, J. Lim, B. Dunn, Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 111, 14925–14931 (2007)
M. Sathiya, A.S. Prakash, K. Ramesha, J.M. Tarascon, A.K. Shukla, V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 133, 16291–16299 (2011)
P. Simon, Y. Gogotsi, B. Dunn, Where do batteries end and supercapacitors begin. Science 343, 1210–1211 (2014)
V. Augustyn, P. Simon, B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014)
Acknowledgements
The financial support from the National Natural Science Foundation of China (Nos. 21301007 and 21301006) and Anhui Normal University Nurturing Project (No. 2016XJJ001) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Yang, Y., He, P. et al. Facile synthesis of MnO2@NiCo2O4 core–shell nanowires as good performance asymmetric supercapacitor. J Mater Sci: Mater Electron 31, 1355–1366 (2020). https://doi.org/10.1007/s10854-019-02649-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02649-3