Abstract
Rutile TiO2 nanorod arrays (NRAs) with average diameter approximately 80 nm were first synthesized by solvothermal method using Ti foil as both titanium source and substrate. And then TiO2@CdS core–shell heterostructure NRAs were fabricated via subsequent successive ionic layer adsorption and reaction (SILAR) route using the TiO2 NRAs as precursor. The thicknesses of CdS shell varied from 4 to 18 nm by changing the times of SILAR cycle. The photocatalytic performances of pure TiO2 and all TiO2@CdS NRAs were investigated on the degradation of rhodamine B (RhB) aqueous solution under simulated sunlight irradiation. Compared to pure TiO2 NRAs, all TiO2@CdS NRAs displayed superior photocatalytic activities, and the optimal CdS shell thickness of TiO2@CdS NRAs was about 11 nm. A possible Z-scheme electron transfer mechanism for TiO2@CdS NRAs nanocomposite with the enhanced photocatalytic performance was provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is well known that water is the source of life, which is an indispensable part of human beings, animals and plants. In recent years, the serious problem of water pollution has been caused by garbage pollution, textile wastewater and other pollution sources [1, 2]. Numerous techniques have been devoted to treat sewage water pollution, such as electrochemical degradation, micellar enhanced ultra-filtration, adsorption or microbial degradation, and photocatalysis [3, 4]. Among them, photocatalysis technology has been regarded as one of the most promising methods in the field of sewage treatment because of the advantages of simple equipment, easy operation, low energy consumption and no secondary pollution [5,6,7].
Titanium dioxide (TiO2), as an important photocatalyst with the advantages of excellent chemical stability, high catalytic reactivity, low cost and non-toxicity, has been widely researched in the field of the sewage water pollution [8,9,10]. There are three mainly distinct crystalline structures of TiO2: anatase, rutile and brookite [11, 12]. Among them, anatase TiO2 (a-TiO2) has been recognized that it exhibits higher photocatalytic activity than other crystalline structures [13]. However, the relatively wide band gap of a-TiO2 (3.2 eV) can only be excited by the UV light, which directly limits the practical application of it in the field of the photocatalysis. [14,15,16]. Recently, some researches indicate that rutile TiO2 (r-TiO2) exhibits unexpectedly properties [17, 18]. In addition, Mohammadpour et al. reported the passivation carrier mobility on the surface of rutile TiO2 was increased by 100-fold [19]. Thakur et al. discussed hydrothermally grown rutile TiO2 has the advantage of optimizing charge transfer with high contact area in halide perovskite solar cells [20]. Therefore, r-TiO2 is of research value in charge transfer and carrier migration, which is also an indispensable advantage for photocatalysis. Furthermore, r-TiO2 with the direct band gap of 3.0 eV can absorb the wavelength of sunlight below 410 nm. This is to say, r-TiO2 can be excited by a part of visible light. Therefore, the research of preparation and photocatalytic activity of the r-TiO2 becomes a research hotspot in these years.
Relatively fast recombination of photogenerated electron–hole pairs is an obvious inherent drawback of conventional single material photocatalyst. Design and preparation of heterojunction composite photocatalyst is an effective strategy for promoting photogenerated carrier separation and transportation [21, 22]. Recent studies manifest that the photocatalytic activity can be enhanced dramatically by coupling TiO2 with other semiconductors, compared to its individual counterpart [23,24,25]. As a typically narrow band gap semiconductor, CdS (2.4 eV) is widely used to prepare the heterojunction composite photocatalyst due to the great visible light absorption ability of it [26, 27]. It has been suggested that the CdS/TiO2 system has a matched band structure and complementary optical properties. At present, the CdS@TiO2 core–shell structure has been obtained through different methods [28, 29]. In contrast, some a-TiO2/CdS composites have also been synthesized [30,31,32], and corresponding experiments results show that the photogenerated carriers easily transfer in TiO2/CdS composite due to the difference in the energy band levels between TiO2 and CdS. However, the preparation and photocatalytic performance of r-TiO2/CdS composites is studied rarely. In addition, TiO2 in the form of film displays obvious virtue in separating from the reaction system and recycling in photocatalytic reaction, compared with other forms of TiO2 [33, 34]. Moreover, it is novel and interesting to study the influence of shell thickness on photocatalytic activity and the effect of Z-scheme heterojunction on the photogenerated carrier transport behavior between interfaces of the nanocomposite [35,36,37]. Therefore, synthesizing r-TiO2@CdS core–shell NRAs with the different CdS shell thicknesses and study of their photocatalytic performances are significant.
In this paper, TiO2@CdS NRAs with different CdS shell thicknesses were fabricated via solvothermal method and subsequent successive ionic layer adsorption and reaction (SILAR) route. The geometrical morphologies, microstructures and optical properties of the pure TiO2 and all TiO2@CdS NRAs were investigated by XRD, SEM, TEM, XPS and UV–Vis DRS. The photocatalytic activities of pure TiO2 and TiO2@CdS NRAs with different CdS shell thicknesses were compared by the photodegradation process of RhB aqueous solution under simulated sunlight irradiation. Furthermore, the possible photocatalytic mechanism was proposed.
2 Experimental section
2.1 Synthesis
Preparation of TiO2 NRAs. HCl is a widely recognized corrosive agent that affects the hydrolysis process of Ti foil to form TiO2. In the hydrothermal process, rutile TiO2 seed layer is influenced by the concentration of HCl on the chemical corrosion of Ti substrate [38]. Rutile TiO2 NRAs were synthesized by solvothermal route using the Ti foil as both substrate and titanium source. The commercial Ti foil with dimension of 9 mm × 9 mm was cleaned by sonication successively in ethanol and deionized water. Then, Ti foil was put into a 30 mL Teflon-lined autoclave with 10 mL of HCl (0.5 mol L−1), and this system was maintained at 180 °C for 24 h. After cooling to room temperature, the film was thoroughly rinsed with deionized water and then dried in a desiccator.
Preparation of TiO2@CdS NRAs. TiO2@CdS core–shell heterostructure NRAs were synthesized by the SILAR way using the as-prepared rutile TiO2 NRAs as precursor. In a typical procedure, the as-synthesized TiO2 NRAs were separately placed into 0.06 mol L−1 Cd(NO3)2 and 0.06 mol L−1 Na2S aqueous solution for 3 min at room temperature with slowly shaking. Cd(NO3)2 and Na2S supply Cd2+ ions and S2− ions, respectively. During each immersion interval, TiO2 NRAs were thoroughly rinsed with deionized water to dislodge the redundant ions from the surface of them. Different CdS shell thicknesses were fabricated via changing the times of SILAR cycle. For brevity, the prepared samples with 3, 6 and 9 times of SILAR cycle were labeled as TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9, respectively. The synthetic procedure of TiO2 and TiO2@CdS NRAs are shown in Scheme 1.
2.2 Characterizations
The crystal structure and phase component were investigated using X-ray diffraction (XRD, Rigaku-D/max-2500 diffractometer, Japan). The geometrical morphologies and microstructures were determined by a field emission scanning electron microscope (FE-SEM, JEOL 7800F, Japan) and transmission electron microscope (TEM, FEI Tenai G2 F20, USA). The elemental compositions and chemical status were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi A1440 system, USA). UV–Vis diffuse reflectance spectra (UV–Vis DRS) were obtained on a spectrometer (Perkin-Elmer Lambda 900, USA) using magnesium oxide as a standard. The Raman spectra of the samples were measured (Horiba-Jobin-Yvon LabRAM ARAMIS). The optical properties were observed by photoluminescence spectra (PL, Renishaw inVia micro-PL spectrometer) at room temperature. Electron spin resonance (ESR) was performed on the ESR spectrometer (Brucker, A300).
2.3 Photocatalytic experiment
Rhodamine B (RhB) water solution was used as a target pollutant to explore the photocatalytic activities of samples under simulated sunlight which was provided by a 300 W Xe lamp (CEL-HXF300). In a typical procedure, the sample was immersed into a rectangular quartz cuvette containing 2 mL RhB water solution (2 mg L−1). Prior to irradiation, the reaction system was placed in dark for 15 min to set up the equilibrium of adsorption–desorption. After a certain period of time, the absorbance spectrum of RhB water solution was analyzed by UV–Vis spectrophotometer (UV-5800PC, China). Repeated photocatalytic tests were executed under the identical experiment circumstance to investigate the reusability of the photocatalyst. The photocatalyst was only washed with deionized water after cycling photocatalytic experiment, and then was used for the next cycle of degradation.
3 Results and discussion
Figure 1 shows the SEM images of pure TiO2 and all TiO2@CdS NRAs. All samples are rod-like and grew on Ti substrate. Figure 1a shows the SEM image of pure TiO2 NRAs. All TiO2 rods display smooth surface and the average diameter is approximately 80 nm. Furthermore, TiO2 nanorods are cubic and the top facets are pyramid. Figures 2b-d show the SEM images of TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9, respectively. Clearly, the diameters of TiO2@CdS NRAs increase with the increasing of SILAR cycle times, and the surfaces of all TiO2@CdS nanorods are getting rough.
Figures 2a, c, e and g show the TEM images of a sole TiO2, TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9 rod, respectively. Those TEM images reveal that the mean length of those nanorods is around 350 nm. The profile of pure TiO2 nanorod is smooth, while the outlines of all TiO2@CdS nanorods are rough. This is in good coincident with the SEM observation from Fig. 1. The HRTEM image of pure TiO2 in Fig. 2b exhibits that the lattice fringes of 0.325 and 0.295 nm are separately consisting with the (110) plane and (001) plane of rutile TiO2. Meanwhile, the HRTEM result also indicates that TiO2 nanorod is single-crystalline nature, and grew along <001> direction. The interplanar distance of 0.206 (Fig. 2d), 0.291 (Fig. 2f, h) and 0.336 nm (Fig. 2f, h) are matching to the (220), (200) and (111) planes of cubic CdS, respectively. In addition, Figs. 2d, f and g show that the CdS shell thickness of TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9 are separately about 4 nm, 11 nm and 18 nm, respectively.
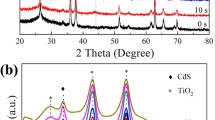
Figure 3 presents the XRD patterns of Ti foil, pure TiO2 and all TiO2@CdS NRAs. Besides some peaks belonging to Ti (JCPDS card No. 87-0713) foil, four main diffraction peaks at 27.4°, 41.2°, 54.3° and 69.0° of pure TiO2 NRAs are observed in Fig. 3b, which are separately corresponding to the (110), (111), (211) and (301) planes of rutile TiO2 (JCPDS card No. 21-1276). Figures 3c–e show the XRD patterns of TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9 NRAs, respectively. No obvious characteristic peaks originating from CdS are detected in Figs. 3c, d, suggesting that the thickness of CdS shell layer are relatively thin in TiO2@CdS-3 and TiO2@CdS-6 NRAs. Three diffraction peaks at about 26.5°, 43.9° and 52.1° in Fig. 3e belonging to (111), (220) and (311) planes of cubic CdS (JCPDS card No. 26-0339) are detected clearly in the sample of TiO2@CdS-9, implying that the thickness of CdS shell layer in TiO2@CdS-9 is higher than other samples. This result confirms the SEM and TEM results.
Since the characteristic peaks of CdS in XRD are not obvious, Raman spectra were used to characterize all samples in order to better explain the composition of phases. The Raman spectra of TiO2 and all TiO2@CdS NRAs are presented in Fig. 4. The characteristic vibration Raman peaks at 246, 437 and 607 cm−1 ascribed to the Eg and A1g active mode vibrations of rutile phase TiO2 [39, 40]. In addition, a clear vibration peak at about 301 cm−1 can be noticed for the TiO2@CdS NRAs, which corresponding to the fundamental longitudinal optical (LO) phonon of CdS cubic phase [41]. Raman spectroscopy results complement the shortcomings of XRD.
Figure 5 shows the XPS spectrum of TiO2@CdS-6 NRAs. The full spectrum in Fig. 5a clearly displays the peaks of Ti, O, Cd, S and C elements. The emergence of C peak originates from the signal of carbon of XPS device [42]. Figure 5b exhibits Ti 2p XPS spectrum. Two peaks located at 458.5 eV and 464.2 eV are assigned to the Ti 2p3/2 and the Ti 2p1/2 binding energies, respectively. The peak splitting between the Ti 2p3/2 and Ti 2p1/2 is 5.7 eV, indicating the existence of Ti4+ state in TiO2@CdS-6 NRAs [43, 44]. Figure 4c shows two main peaks at 529.7 eV and 532.1 eV. The first peak originates from crystal lattice oxygen of TiO2 [45, 46]. And the second peak belongs to oxygen in hydroxyl groups (–OH) [47]. As shown in Figs. 4d, the binding energies of Cd 3d5/2 and Cd 3d3/2 located at 405.1 eV and 411.8 eV, respectively, indicating that Cd exists in the form of Cd2+ [48,49,50]. For the spectrum of S 2p (in Fig. 4e), two peaks at 161.3 eV and 162.8 eV are separately accounted for S 2p3/2 and S 2p1/2, indicating that S element existed in the form of S2− [51, 52]. In short, the XPS result further confirms that Ti, O, Cd and S elements exist simultaneously in TiO2@CdS-6 NRAs.
Figure 6 shows the UV–Vis DRS of pure TiO2, CdS nanoparticle, TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9 NRAs. Pure TiO2 NRAs exhibit absorption in the wavelength below 410 nm due to the intrinsic band gap of rutile TiO2. The UV–Vis DRS of TiO2@CdS-3 and pure TiO2 NRAs are similar. This is because the thickness of CdS shell layer in TiO2@CdS-3 NRAs is very thin. Compared to pure TiO2 NRAs, the absorption of TiO2@CdS-6 and TiO2@CdS-9 NRAs has a weak red shift. Furthermore, both TiO2@CdS-6 and TiO2@CdS-9 display obviously enhanced light absorption abilities in the visible region comparing to pure TiO2 NRAs. The absorption intensity in the visible region of TiO2@CdS-9 is stronger than that of TiO2@CdS-6 owing to the relatively thick CdS shell layer in TiO2@CdS-9 NRAs. The band gap (Eg) value is calculated on the basis of the formula: αhν = (hν − Eg)n [53]. As shown in Fig. 6b, the Eg values of TiO2, CdS, TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9 are 3.02, 2.15, 3.01, 2.99 and 2.95 eV, respectively. This indicates that these Eg values of TiO2@CdS core–shell heterostructure NRAs strongly depend on the CdS shell thickness.
Photoluminescence (PL) technology is an important means to study semiconductor impurities and defects, and has important application value in the field of photocatalysis. Figure 7 showed PL spectra of all samples excited in 325 nm. The main emission peaks of these samples cover 500–600 nm region. And the deep-level emission came from the surface defects of samples [54]. The fluorescence intensity in Fig. 7 shows that TiO2@CdS-6 has the highest PL intensity. Obviously, it is reasonable to modify TiO2 NRAs by CdS, which can drastically increase the PL intensity of pure TiO2. The results show that the higher the defect concentration, the capture of photoelectrons leads to the improvement of carrier separation efficiency. That is to say, with a high surface defect concentration of CdS, it can be used as an electron trap and photocatalytic reactive site to capture part of the electrons transported by TiO2 and participate in the reaction, and the other part is involved in luminescence. Therefore, the higher defect concentration is beneficial to the photocatalytic reaction [55, 56].
In order to prove that the heterojunction between the TiO2 and CdS is benefit for the photocatalytic performance, the TiO2@SiO2 and TiO2@SiO2@CdS-6 NRAs were prepared and the SEM images of these nanocomposites were shown in Figs. S1 and S2. And the photocatalytic activity of TiO2@CdS was compared with pure TiO2 and TiO2@SiO2@CdS NRAs. In the photocatalytic experiment, SiO2 is inert and the CdS becomes the bare layer in TiO2@SiO2@CdS-6. The photocatalytic activities of TiO2, TiO2@SiO2@CdS-6, and all TiO2@CdS NRAs are evaluated by the photodegradation experiments of RhB aqueous solution under simulated solar light irradiation, and the corresponding results are shown in Fig. 8a. Obviously, blank experiment reveals that the photolysis of RhB aqueous solution can be omitted in the absence of photocatalyst. After 120 min irradiation, 38.0% and 53.1% RhB water solution are photodegraded by pure TiO2 NRAs and TiO2@SiO2@CdS-6, respectively. Whereas 84.7%, 96.6% and 92.2% RhB water solutions is photodegraded using TiO2@CdS-3, TiO2@CdS-6 and TiO2@CdS-9 as photocatalyst, respectively. The photocatalytic activities of the prepared samples under simulated solar light irradiation show the following order: TiO2@CdS-6 > TiO2@CdS-9 > TiO2@CdS-3 > TiO2@SiO2@CdS-6 > TiO2. Namely, all TiO2@CdS NRAs exhibit higher photocatalytic activity than pure TiO2 NRAs and TiO2@SiO2@CdS-6. In addition, the photocatalytic activities of TiO2@CdS NRAs strongly depend on the thickness of CdS shell.
The reaction rate constant (k, min−1) is investigated via the first order kinetic kinetics equation ln(C0/C) = kt [57, 58]. And C0 and C on behalf of the real-time and initial concentrations of RhB, respectively. Figure 8b illustrates the k values of TiO2 and all TiO2@CdS NRAs. It is quite clear that TiO2@CdS-6 NRAs owns the biggest k value among all as-synthesized samples, while the pure TiO2 NRAs possesses the smallest k value. And the k value of TiO2@CdS-6 NRAs is around 6.6 and 3.6 times than that of pure TiO2 NRAs and TiO2@SiO2@CdS-6 NRAs, respectively. As shown in Fig. 8c, we can notice that the efficiency of the TiO2@CdS-6 degradation curve for RhB dye ranges from 0 to 120 min under simulated solar light irradiation. The true color change of RhB aqueous are inset in Fig. 8c. As the reaction time goes on, the color of RhB aqueous solution becomes lighter. The reusability of TiO2@CdS-6 NRAs is evaluated in recycling reaction. The corresponding result is shown in Fig. 8b. According to Fig. 8b, the photocatalytic efficiency reaches to 93.7% after five successive cycles, demonstrating that TiO2@CdS-6 NRAs exhibit good reusability. This result also indicates that CdS nanoparticles are firmly attached to TiO2 nanorods.
It is widely accepted that mainly three reactive species including hydroxyl radicals (·OH), photogenerated hole (h+) and superoxide anion radical (·O2−) are involved during the photocatalytic reaction. In order to explore the mechanism of photodegradation reaction, the trapping experiments of reactive species are executed using the TiO2@CdS-6 NRAs as photocatalyst. Three quenchers, ethylenediaminetetraacetate (EDTA), tert-butyl alcohol (t-BuOH) and benzoquinone (BQ) are employed to capture h+, ·OH, and ·O2−, respectively [59,60,61]. The result in Fig. 9 shows that three quenchers lead to the fast deactivation of TiO2@CdS-6 NRAs. After adding BQ, EDTA and t-BuOH, the degradation rate of RhB water solution decreases from 96.6% to 36.0% (BQ), 24.2% % (EDTA) and 42.9% (t-BuOH), respectively, indicating that ·O2−, h+ and ·OH play the important roles in the photocatalytic process.
Electron spin resonance (ESR) spectra were applied to further investigate the superoxide and hydroxyl radicals generated under simulated solar light. 5,5-Dimethyl-1-pyrroline N-oxide (DMPO), as a trap agent, is dispersed in a methanol and water solution [35, 62]. As illustrated in Fig. 10a, b, no obvious DMPO–·O2− and DMPO–·OH signals are detected under darkness. However, characteristic peaks of DMPO–·O2− and DMPO–·OH were observed under simulated solar light irradiation. This indicating that the presence of ·O2− and ·OH radicals after simulated solar light irradiation. The ESR analysis is consistent with the results of species trapping experiments.
The superior photocatalytic activity of TiO2@CdS NRAs is attributed to the synergistic effect of TiO2 and CdS, which is favorable for the segregation of photoinduced electron–hole pairs. Figure 11 illustrates the possible photocatalytic mechanism of TiO2@CdS NRAs, and there are two possible ways for the transfer of photoinduced carriers. Under simulated sunlight illumination, both TiO2 and CdS can be excited to produce photoinduced electrons and holes. Usually, the photoinduced electrons from CdS will migrate to TiO2, and the holes of TiO2 will move to CdS because of the stepwise band edge structure in TiO2@CdS heterostructure, as shown the mechanism a in Fig. 11. However, the photoinduced electrons of TiO2 cannot reduce O2 to form ·O2− because the conduction band (CB) of TiO2 is lower than the standard redox potential of O2/·O2−. Similarly, photoinduced holes on CdS surface cannot oxidize H2O to produce ·OH radicals because the valence band (VB) of CdS is higher than the standard potential of OH−/·OH and H2O/·OH [63]. According to the trapping experiments of reactive species, h+, ·OH and ·O2− are involved during the photocatalytic reaction. So, the photocatalytic reaction obeys the mechanism b rather than mechanism a in Fig. 11. This is to say, the photoinduced electrons in CdS tend to keep in the CB of CdS, and the photoinduced holes in TiO2 remain in the VB of TiO2. Meanwhile, the photoinduced electrons in the CB of TiO2 combine with the photoinduced holes in VB of CdS. This is the typical Z-scheme mechanism [32, 64]. In this case, the recombination of photoinduced electron–hole pairs are impeded efficiently and the lifetime of charge carrier in TiO2@CdS core–shell heterostructure is prolonged. Consequently, the photocatalytic activity of TiO2@CdS heterostructure NRAs is higher than that of pure TiO2 NRAs. In addition, TiO2@CdS-6 NRAs exhibits the best photocatalytic activity among all TiO2@CdS NRAs. Namely, the optimal thickness of CdS shell is 11 nm. TiO2@CdS-3 NRAs shows the worse photocatalytic activity than TiO2@CdS-6 NRAs because the thickness of CdS shell layer in TiO2@CdS-3 NRAs is relatively thin. For TiO2@CdS-9 NRAs, the excessively thick CdS shell not only hinders the TiO2 absorption of sunlight but also restrains the migration of charge carriers between CdS and TiO2 in TiO2@CdS NRAs heterostructure.
4 Conclusions
Core–shell structure TiO2@CdS nanorod arrays with different CdS shell thicknesses were fabricated via solvothermal way and subsequent successive ionic layer adsorption and reaction (SILAR) method. The photocatalytic result showed that the photocatalytic activities of all TiO2@CdS NRAs were drastically enhanced compared to that of pure TiO2 NRAs under simulated sunlight irradiation. The Z-scheme heterostructure formed by TiO2@CdS promotes the transport and separation of photogenerated carriers between the interfaces of the two materials, greatly improving the photocatalytic efficiency. Among all TiO2@CdS NRAs, the thickness CdS shell of 11 nm exhibited the highest photocatalytic activity and good reusability, and their degradation rate constant were about 6.6 times than that of pure TiO2.
References
Z. Zhu, Z.Y. Lu, D.D. Wang, X. Tang, Y.S. Yan, Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity. Appl. Catal. B 182, 115–122 (2016)
D.J. Martin, K. Qiu, S.A. Shevlin, A.D. Handoko, X. Handoko, Highly efficient photocatalytic h2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew. Chem. Int. Ed. 53, 9240–9245 (2014)
M. Rafatullah, O. Sulaiman, R. Hashim, A. Ahmad, Adsorption of methylene blue on lowcost adsorbents: a review. J. Hazard. Mater. 177, 70–80 (2010)
C. Namasivayam, D. Kavitha, Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigm. 54, 47–58 (2002)
J. Zhang, L. Huang, H. Jin, Y. Sun, X. Ma, Constructing two-dimension MoS2/Bi2WO6 core-shell heterostructure as carriers transfer channel for enhancing photocatalytic activity. Mater. Res. Bull. 85, 140–146 (2017)
Z.F. Jiang, D.L. Jiang, Z.X. Yan, D. Liu, A new visible light active multifunctional ternary composite based on TiO2-In2O3 nanocrystals heterojunction decorated porous graphitic carbon nitride for photocatalytic treatment of hazardous pollutant and H2 evolution. Appl. Catal. B 170, 195–205 (2015)
X. Li, T. Xia, C.H. Xu, J. Murowchick, X.B. Chen, Synthesis and photoactivity of nanostructured CdS-TiO2 composite catalysts. Catal. Today 225, 64–73 (2014)
X. Chen, S.S. Mao, Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007)
C. Zhao, H. Luo, F. Chen, P. Zhang, L. Yi, K. You, A novel composite of TiO2 nanotubes with remarkably high efficiency for hydrogen production in solardriven water splitting. Energ. Environ. Sci. 7, 1700–1707 (2014)
C.B.D. Marien, T. Cottineau, D. Roberta, P. Drogui, TiO2 nanotube arrays: influence of tube length on the photocatalytic degradation of Paraquat. Appl. Catal. B 194, 1–6 (2016)
D. Reyes-Coronado, G. Rodr´ıguez-Gattorno, M.E. Espinosa-Pesqueira, C. Cab, R. Coss, G. Oskam, Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology 19, 145605 (2008)
M. Dawson, C. Ribeiro, M.R. Morelli, Rutile supported anatase nanostructured films as photocatalysts for the degradation of water contaminants. Ceram. Int. 42, 808–819 (2016)
K. Ozawa, S. Yamamoto, R. Yukawa, R.Y. Liu, N. Terashima, Y. Natsui, H. Kato, K. Mase, I. Matsuda, Correlation between photocatalytic activity and carrier lifetime: acetic acid on single-crystal surfaces of anatase and rutile TiO2. J. Phys. Chem. C 122, 9562–9569 (2018)
X. Yang, L. Wu, L. Ma, X. Li, T. Wang, S. Liao, Pd nano-particles (NPs) confined in titanate nanotubes (TNTs) for hydrogenation of cinnamaldehyde. Catal. Commun. 59, 184–188 (2015)
R. Daghrir, P. Drogui, D. Robert, Modified TiO2 for environmental photocatalytic applications: a review. Ind. Eng. Chem. Res. 52, 3581–3599 (2013)
P. Kar, S. Zheng, Y. Zhang, E. Vahidzadeh, A. Manuel, R. Kisslinger, K. Alam, U. Thakur, N. Mahdi, P. Kumar, K. Shankar, High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Appl. Catal. B 243, 522–536 (2019)
M. Kobielusz, K. Pilarczyk, E. Swietek, K. Szaciłowski, W. Macyk, Spectroelectrochemical analysis of TiO2 electronic states-implications on the photocatalytic activity of anatase and rutile. Catal. Today 309, 35–42 (2018)
U. Nwankwo, R. Bucher, A.B.C. Ekwealor, M. Maaza, F.I. Ezema, Synthesis and characterizations of rutile-TiO2 nanoparticles derived from chitin for potential photocatalytic applications. Vacuum 161, 49–54 (2019)
A. Mohammadpour, B.D. Wiltshire, Y. Zhang, S. Farsinezhad, A.M. Askar, R. Kisslinger, Y. Ren, P. Kar, K. Shankar, 100-fold improvement in carrier drift mobilities in alkanephosphonate-passivated monocrystalline TiO2 nanowire arrays. Nanotechnology 28, 144001 (2017)
U.K. Thakur, A.M. Askar, R. Kisslinger, B.D. Wiltshire, P. Kar, K. Shankar, Halide perovskite solar cells using monocrystalline TiO2 nanorod arrays as electron transport layers: impact of nanorod morphology. Nanotechnology 28, 274001 (2017)
D. Sarkar, C.K. Ghosh, S. Mukherjee, Three dimensional Ag2O/TiO2 type-II (p-n) nanoheterojunctions for superior photocatalytic activity. ACS Appl. Mater. Interfaces. 5, 331–337 (2013)
Y.S. Chang, M. Choi, M. Baek, P.Y. Hsieh, K.J. Yong, Y.J. Hsu, CdS/CdSe co-sensitized brookite H: TiO2 nanostructures: charge carrier dynamics and photoelectrochemical hydrogen generation. Appl. Catal. B 225, 379–385 (2018)
T. Lv, L.K. Pan, X.J. Liu, Z. Sun, Visible-light photocatalytic degradation of methyl orange by CdS-TiO2-Au composites synthesized via microwave-assisted reaction. Electrochim. Acta 83, 216–220 (2012)
N. Mohaghegh, B. Eshaghi, E. Rahimi, M.R. Gholami, Ag2CO3 sensitized TiO2 nanoparticles prepared in ionic liquid medium: a new Ag2CO3/TiO2/RTIL heterostructure with highly efficient photocatalytic activity. J. Mol. Catal. A 406, 152–158 (2015)
J. Zhang, X.M. Ma, L.L. Zhang, Z.D. Lu, Constructing a novel n-p-n dual heterojunction between anatase TiO2 nanosheets with coexposed 101}, {001 facets and porous ZnS for enhancing photocatalytic activity. J. Phys. Chem. C 121, 6133–6140 (2017)
Y.B. Liu, H.B. Zhou, B.X. Zhou, J.H. Li, H.C. Chen, J.J. Wang, J. Bai, Highly stable CdS-modified short TiO2 nanotube array electrode for efficient visible-light hydrogen generation. Int. J. Hydrog. Energy 36, 167–174 (2011)
Y. Lin, J. Song, Y. Ding, S. Lu, Z.L. Wang, Alternating the output of a CdS nanowire nanogenerator by a white-light-stimulated optoelectronic effect. Adv. Mater. 20, 3127–3130 (2008)
Y. Liu, P. Zhang, B.Z. Tian, J.L. Zhang, Enhancing the photocatalytic activity of CdS nanorods for selective oxidation of benzyl alcohol by coating amorphous TiO2 shell layer. Catal. Commun. 70, 30–33 (2015)
W.H. Dong, F. Pan, L.L. Xu, M.R. Zheng, C.H. Sow, K. Wu, G.Q. Xu, W. Chen, Facile synthesis of CdS@TiO2 core-shell nanorods with controllable shell thickness and enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 349, 279–286 (2015)
C. Su, C. Shao, Y. Liu, Electrospun nanofibers of TiO2/CdS heteroarchitectures with enhanced photocatalytic activity by visible light. J. Colloids Interface Sci. 359, 220–227 (2011)
X.Y. Guo, C.F. Chen, W.Y. Song, X. Wang, W.H. Di, W.P. Qin, CdS embedded TiO2 hybrid nanospheres for visible light photocatalysis. J. Mol. Catal. A 387, 1–6 (2014)
P. Gao, J. Liu, T. Zhang, D.D. Sun, W. Ng, Hierarchical TiO2/CdS “spindle-like” composite with high photodegradation and antibacterial capability under visible light irradiation. J. Hazard. Mater. 229–230, 209–216 (2012)
Y. Zhang, Y. Gao, X.H. Xia, Q.R. Deng, M.L. Guo, Structural engineering of thin films of vertically aligned TiO2 nanorods. Mater. Lett. 64, 1614–1617 (2010)
H. Pan, J.S. Qian, A. Yu, M.G. Xu, TiO2 wedgy nanotubes array flims for photovoltaic enhancement. Appl. Surf. Sci. 257, 5059–5063 (2011)
Y.T. Xue, Z.S. Wu, X.F. He, X. Yang, X.Q. Chen, Z.Z. Gao, Constructing a Z-scheme heterojunction of egg-like core@shell CdS@TiO2 photocatalyst via a facile reflux method for enhanced photocatalytic performance. Nanomaterials 9, 222 (2019)
C.J. Liu, Y.H. Yang, J. Li, S. Chen, Phase transformation synthesis of TiO2/CdS heterojunction film with high visible-light photoelectrochemical activity. Nanotechnology 29, 265401 (2018)
E. Üzer, P. Kumar, R. Kisslinger, P. Kar, U.K. Thakur, S. Zeng, K. Shankar, T. Nilges, Vapor deposition of semiconducting phosphorus allotropes into TiO2 nanotube arrays for photoelectrocatalytic water splitting. ACS Appl Nano Mater 26, 3358–3367 (2019)
M. Xi, Y.L. Zhang, L.Z. Long, X.J. Li, Controllable hydrothermal synthesis of rutile TiO2 hollow nanorod arrays on TiCl4 pretreated Ti foil for DSSC application. J. Solid State Chem. 219, 118–126 (2014)
P. Kumar, U.K. Thakur, K. Alam, P. Kar, R. Kisslinger, S. Zeng, S. Patel, K. Shankar, Arrays of TiO2 nanorods embedded with fluorine doped carbon nitride quantum dots (CNFQDs) for visible light driven water splitting. Carbon 137, 174–187 (2018)
P. Kar, Y. Zhang, S. Farsinezhad, A. Mohammadpour, B.D. Wiltshire, H. Sharma, K. Shankar, Rutile phase n-and p-type anodic titania nanotube arrays with square-shaped pore morphologies. Chem. Commun. 51, 7816–7819 (2015)
S.S. Mali, S.K. Desai, D.S. Dalavi, C.A. Betty, P.N. Bhosalea, P.S. Patil, CdS sensitized TiO2 nanocorals: hydrothermal synthesis, characterization, application. Photochem. Photobiol. Sci. 10, 1652–1658 (2011)
P.P. Zhou, Y. Xie, J. Fang, Y. Ling, C.L. Yu, X.M. Liu, Y.H. Dai, Y.C. Qin, D. Zhou, CdS quantum dots confined in mesoporous TiO2 with exceptional photocatalytic performance for degradation of organic pollutants. Chemosphere 178, 1–10 (2017)
N. Qin, J.H. Xiong, R.W. Liang, Y.H. Liu, S.Y. Zhang, Y.H. Li, Z.H. Li, L. Wu, Highly efficient photocatalytic H2 evolution over MoS2/CdS-TiO2 nanofibers prepared by an electrospinning mediated photodeposition method. Appl. Catal. B 202, 374–380 (2017)
S. David, M.A. Mahadik, H.S. Chung, J. Ryu, J.S. Jang, Facile hydrothermally synthesized a novel CdS nanoflower/rutile-TiO2 nanorod heterojunction photoanode used for photoelectrocatalytic hydrogen generation. ACS Sustain. Chem. Eng. 5, 7537–7548 (2017)
S.Y. Li, Z.L. Liu, G.X. Xiang, B.H. Ma, X.D. Meng, Y.L. He, Influence of calcination temperature on the photocatalytic performance of the hierarchical TiO2 pinecone-like structure decorated with CdS nanoparticles. Ceram. Int. 45, 767–776 (2019)
Y.F. Li, L.L. Wang, Z.L. Li, Y.L. Liu, Z.Y. Peng, Synthesis and photocatalytic property of V2O5@TiO2 core-shell microspheres towards gaseous benzene. Catal. Today 321–322, 164–171 (2019)
H.Y. Yang, Z.L. Liu, K. Wang, S.T. Pu, S.N. Yang, L. Yang, A facile synthesis of TiO2-CdS heterostructures with enhanced photocatalytic activity. Catal. Lett. 147, 2581–2591 (2017)
P.S. Shinde, J.W. Park, M.A. Mahadik, J. Ryu, J.H. Park, Y.J. Yi, J.S. Jang, Fabrication of efficient CdS nanoflowers-decorated TiO2 nanotubes array heterojunction photoanode by a novel synthetic approach for solar hydrogen production. Int. J. Hydrog. Energy 46, 21078–21087 (2016)
G.D. Yang, B.L. Yang, T.C. Xiao, Z.F. Yan, One-step solvothermal synthesis of hierarchically porous nanostructured CdS/TiO2 heterojunction with higher visible light photocatalytic activity. Appl. Surf. Sci. 283, 402–410 (2013)
M. Fujishima, Y. Nakabayashi, K. Takayama, H. Kobayashi, H. Tada, High coverage formation of CdS quantum dots on TiO2 by the photocatalytic growth of preformed seeds. J. Phys. Chem. C 120, 17365–17371 (2016)
X.Y. Li, D.X. Liu, Z. Shi, J.H. Yang, Effect of Ag2S shell thickness on the photocatalytic properties of ZnO/Ag2S core-shell nanorod arrays. J. Mater. Sci. 54, 1226–1235 (2019)
X.Y. Li, X. Li, B.Y. Zhu, J.S. Wang, H.X. Lan, X.B. Chen, Synthesis of porous ZnS ZnO and ZnS/ZnO nanosheets and their photocatalytic properties. RSC Adv. 7, 30956–30962 (2017)
Y.H. Zhang, N. Zhang, Z.R. Tang, Y.J. Xu, Identification of Bi2WO6 as a highly selective visible-light photocatalyst toward oxidation of glyceroltodihy-droxyacetone in water. Chem. Sci. 4, 1820–1824 (2013)
C.L. Cao, C.G. Hu, W.D. Shen, S.X. Wang, Y.S. Tian, X. Wang, Synthesis and characterization of TiO2/CdS core–shell nanorod arrays and their photoelectrochemical property. J. Alloys Compd. 523, 139–145 (2012)
X. Li, C.Y. Liu, D.Y. Wu, J.Z. Li, P.W. Huo, H.Q. Wang, Improved charge transfer by size-dependent plasmonic Au on C3N4 for efficient photocatalytic oxidation of RhB and CO2 reduction. Chin. J. Catal. 40, 928–939 (2019)
D. Li, C. Shen, J.Z. Liu, Y.J. Li, X.H. Zhou, P.W. Song, H.Q. Huo, Y.S. Wang, Yan, Fabricated rGO-modified Ag2S nanoparticles/g-C3N4 nanosheets photocatalyst for enhancing photocatalytic activity. J. Colloids Interface Sci. 554, 468–478 (2019)
Y.Y. Lu, Y.Y. Zhang, J. Zhang, Y. Shi, Z. Li, Z.C. Feng, C. Li, In situ loading of CuS nanoflowers on rutile TiO2 surface and their improved photocatalytic performance. Appl. Surf. Sci. 370, 312–319 (2016)
X.Y. Li, D.X. Liu, B.Y. Zhu, J. Wang, J.H. Lang, Facile preparation of ZnO/Ag2CO3 heterostructured nanorod arrays with improved photocatalytic activity. J. Phys. Chem. Solids 125, 96–102 (2019)
D.D. Wang, D.L. Han, Z. Shi, J. Wang, J.H. Yang, X.Y. Li, H. Song, Optimized design of three-dimensional multi-shell Fe3O4/SiO2/ZnO/ZnSe microspheres with type II heterostructure for photocatalytic applications. Appl. Catal. B 227, 61–69 (2018)
H.R. Liu, H.F. Zhai, C.J. Hu, J.E. Yang, Z.Y. Liu, Hydrothermal synthesis of In2O3 nanoparticles hybrid twins hexagonal disk ZnO heterostructures for enhanced photocatalytic activities and stability. Nanoscale Res. Lett. 12, 466–476 (2017)
H.R. Liu, Y.C. Hu, Z.X. Zhang, X.G. Liu, H.S. Jia, B.S. Xu, Synthesis of spherical Ag/ZnO heterostructural composites with excellent photocatalytic activity under visible light and UV irradiation. Appl. Surf. Sci. 355, 644–652 (2015)
H.X. Zhao, S. Cui, L. Yang, G.D. Li, N. Li, X.T. Li, Synthesis of hierarchically meso-macroporous TiO2/CdS heterojunction photocatalysts with excellent visible-light photocatalytic activity. J. Colloids Interface Sci. 23, 4246–4254 (2013)
W. Wu, C.Z. Jiang, V.A.L. Roy, Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 7, 38–58 (2015)
Y.X. Zhu, Y.F. Wang, Z. Chen, L.S. Qin, L.B. Yang, L. Zhu, Visible light induced photocatalysis on CdS quantum dots decorated TiO2 nanotube arrays. Appl. Catal. A 498, 164–171 (2015)
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 61378085) and the Thirteenth Five-Year Program for Science and Technology of Education Department of Jilin Province (Item No. JJKH20191017KJ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, Z., Liu, J., Lan, H. et al. Effect of CdS shell thickness on the photocatalytic properties of TiO2@CdS core–shell nanorod arrays. J Mater Sci: Mater Electron 30, 17682–17692 (2019). https://doi.org/10.1007/s10854-019-02118-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02118-x