Abstract
Li2FeSiO4/C is successfully modified by Li3PO4 additive to achieve excellent electrochemical properties. In comparison with bare Li2FeSiO4/C, the samples with Li3PO4 show no changes in the morphology and structure, which are verified by X-ray powder diffraction and scanning electron microscopy results. High resolution transmission electron microscopy image confirms that Li2FeSiO4/C and Li3PO4 exist in the form of nanocomposites. When used as LIB cathodes, the Li2FeSiO4/Li3PO4/C composites show notably improved electrochemical performance with outstanding high rate performance and superior cyclability than those of Li2FeSiO4/C. The sample with 6 mmol% (LFS-6) exhibits a large discharge capacity of 114.1 mAh g−1 with a remarkable capacity retention of 104.2% over 100 cycles at 10 C, which are much better than those of Li2FeSiO4/C (89.0 mAh g−1 and capacity retention of 75.9%). The enhancement in the electrochemical properties of Li2FeSiO4/C may be attributed to the existence of Li3PO4 additive with high ionic conductivity, which can accelerate the lithium ions diffusion capability and facilitate the charge transfer process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rechargeable lithium-ion batteries (LIBs) have been highlighted for their large scale application of electronic portable devices and electric vehicles [1,2,3]. High capacity, high energy and power densities, low cost and lifespan are the key factors in practical applications of LIBs [4, 5]. Recently, extensive attention has been focused on lithium iron orthosilicate (Li2FeSiO4) because of their distinct advantages such as high security performance, obvious environmental benignity and low cost owing to the abundant resources of Fe and Si [6]. Additionally, Li2FeSiO4 shows high theoretical capacity (> 330 mAh g−1) as a result of extracting two Li+ ions per formula unit [7, 8]. However, like most polyanion-based cathode materials, Li2FeSiO4 also suffers from very low electronic conductivity (~ 6 × 10−14 S cm−1) [8, 9] together with slow lithium ion diffusion rate (~ 1 × 10−14 cm2 S−1) [10], thus leading to extremely poor rate capability.
To solve these problems, several attempts have been made. Metal ion doping has been reported to be a suitable way to enhance the electrochemical properties of Li2FeSiO4 for the improved electronic conductivity and accelerated lithium ion diffusion caused by lattice defects [11,12,13,14]. Yi et al. [11] reported that Li2FeSiO4/C doped with 5% Mn showed excellent electrochemical performance with highest discharge capacity (248.5 mAh g−1 at 0.1 C) in the first cycle, outstanding high-rate performance and high value of capacity retention. Morphology control has also been illustrated to exert positive effects on the electrochemical properties of the material [15,16,17,18,19]. Varieties of shapes of Li2FeSiO4 have been investigated, such as shuttle-like [15], flower-like [16] and nanoworm-like shapes [17] as well as hollow spheres [18] and three-dimensionally ordered macroporous structure [19]. Moreover, Li2FeSiO4 coated with carbon has been confirmed to achieve larger discharge capacity and better cycling performance [20,21,22]. It is worth noting that the as-synthesized composites coated with a thin carbon layer can not only increase the electronic conductivity, but also control the particle size of material during the modification process [23, 24].
Nowadays, coating with fast ion conductor has been a widely used modified method. Various active materials such as Li2SiO3 [25], Fe7SiO10 [26] and AlPO4 [27] have been coated on the surface of Li2FeSiO4, which greatly improve the rate performance, especially the high rate performance in comparison with the original material. For instance, Li et al. [26] synthesized Li2FeSiO4/Fe7SiO10/C via a sol–gel method by adjusting the molar ratio of Fe and Si. Compared with Li2FeSiO4/C, the resulting composite showed much better high-rate performance with discharge capacities of 128 and 112 mAh g−1, respectively, at 5 and 10 C. As a fast lithium ion conductor, Li3PO4 exhibits unique advantages with ionic conductivity about 10−6~10−8 S cm−1 [28, 29]. By the virtue of high ionic conductivity and strong P=O bond of Li3PO4, the migration rate of Li+ between electrode and electrolyte is expected to be increased in a certain extent [30]. Importantly, Li3PO4 provides the possibility to reduce the corrosion of electrolyte to electrode materials when used as a coating material [31]. Hence, Li3PO4 has been commonly used to enhance the cyclability and rate performance of some cathodes such as LiCoO2 [32], Li(Ni0.4Co0.3Mn0.3)O2 [31], LiNi0.5Mn1.5O4 [33], LiFePO4 [34], LiMn2O4 [35] and Li-rich oxide [36,37,38,39].
In the present work, Li3PO4 has been introduced into Li2FeSiO4/C as a property improvement additive. The effects of different amounts of Li3PO4 on the structure, morphology and electrochemical performances of cathodes have been systematically investigated. By introducing Li3PO4, the rate performance of Li2FeSiO4/C can be significantly improved with nearly no capacity decay.
2 Experimental
2.1 Synthesis of Li3PO4
The Li3PO4 powder was precipitated by adding H3PO4 (85% solution) dropwise into 0.4 M LiOH aqueous solution with a molar ratio of Li: P equal to 3:1. The resulting white precipitates were washed with distilled water and dried at 120 °C. Finally, the Li3PO4 was obtained by ball-milling the dried products for 3 h.
2.2 Synthesis of Li2FeSiO4/C composite
Li2FeSiO4/C composite was prepared by a modified sol–gel method according to Ref. [40]. Firstly, 2 g poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide) triblock copolymer P123 (EO20PO70EO20) was dissolved in 40 mL absolute alcohol, and the stoichiometric ratio of CH3COOLi·2H2O and Fe(NO3)3·9H2O were added into alcohol solution in order under vigorous magnetic stir. The solution was stirred and dissolved to get a uniform and transparent red-brown solution. Then the corresponding amount of TEOS was added to the mixture. The red gel was obtained under vigorous magnetic stir at 50 °C and dried at 100 °C for 12 h. Finally, the dry gel was annealed at 600 °C for 10 h under argon atmosphere. After cooling to room temperature, the products were obtained and marked as LFS.
2.3 Synthesis of Li2FeSiO4/Li3PO4/C composites
The synthesized Li3PO4 was added into the solution containing P123, CH3COOLi·2H2O and Fe(NO3)3·9H2O according to the given molar ratio, and the stoichiometric ratio of TEOS was dissolved in the above solution. The subsequent processing was the same as the process of Li2FeSiO4/C. For comparison, the molar percent of Li3PO4 to Li2FeSiO4 were 4%, 6%, 8% and 10%, respectively. The samples were marked as LFS-4, LFS-6, LFS-8 and LFS-10.
2.4 Characterization
The phase structure of as-synthesized samples was conducted by powder X-ray diffraction (Bruker D8 Advance, Germany) using Cu Kα. The morphologies and microstructures of samples were observed by scanning electron microscope (Zeiss SIGMA FESEM) and Transmission electron microscope (JEM-2100F, JEOL Ltd.). The carbon contents in the Li2FeSiO4/Li3PO4/C composites and Li2FeSiO4/C were characterized by VARIOELIII elemental analyzer (Elementar Analysen System GmbH, Germany).
2.5 Electrochemical tests
The Li2FeSiO4/Li3PO4/C composites and Li2FeSiO4/C, acetylene black and polytetrafluoroethylene (PTFE) binder were mixed in proportion at a ratio of 75:20:5 (in mass, wt%), and a proper amount of isopropanol was added as the dispersant. The mixture was made into paste in the mortar and transferred to the rolling machine to make film. The film was cut into disks about 1 cm in diameter and coated on stainless steel, after vacuum drying at 60 °C. In an Ar-filled glovebox, batteries were assembled using lithium foil, Celgard 2300 microporous films, 1 M LiPF6 in the ethylene carbonate (EC) and dimethyl carbonate (DMC) (1:1 v/v) as anodes, separators and electrolytes, respectively. The active materials were assembled into CR2016 coin-type cell for electrochemical measurements. Galvanostatically charge–discharge tests were done between 1.5 V and 4.8 V using battery test system (Neware, China). And all the specific capacities were calculated with the mass of Li2FeSiO4/Li3PO4/C composites and Li2FeSiO4/C, not including carbon content. Electrochemical impedance spectroscopies (EIS) were tested with an AC amplitude of 5 mV in the applied frequency of 0.01 Hz to 100 kHz via CHI760C electrochemistry workstation.
3 Results and discussion
Table 1 lists the carbon contents in Li2FeSiO4/Li3PO4/C composites and Li2FeSiO4/C, which were characterized by elemental analyzer. Obviously, carbon contents in all the composites are very close to each other.
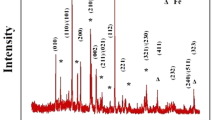
The XRD pattern and SEM image of the prepared Li3PO4 are presented in Fig. 1. Figure 1a displays that all the diffraction peaks match well with the orthogonal phase Li3PO4 (JCPDS NO. 71-1528). There are no impurities can be found, demonstrating that the obtained sample is pure phase. Moreover, the peak type of the sample is relatively sharp and the base is flat, suggesting the high crystallinity of the synthesized sample. From SEM image (Fig. 1b), it is clear that the particles of Li3PO4 after ball milling are uniform and the average diameter is about 40 nm.
Figure 2 illustrates the XRD pattern and SEM image of Li2FeSiO4/C. As can be seen in Fig. 2a, the reflection peaks can be assigned to the monoclinic structure Li2FeSiO4 with a space group of P21/n [41]. No impurity peaks can be detected in the XRD pattern, suggesting the high purity of prepared sample. Meanwhile, no reflection peaks associated with carbon can be seen, which may be related to the amorphous property of carbon. From SEM image (In Fig. 2b), we can see that the nanoparticles are uniformly distributed and the average diameter is around 30 nm.
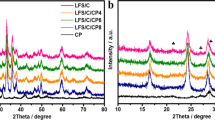
The XRD patterns of the samples with different contents of Li3PO4 (LFS-4, LFS-6, LFS-8 and LFS-10) are displayed in Fig. 3. The main reflection peaks of the samples with Li3PO4 are the same as those of Li2FeSiO4/C expect the Li3PO4 peaks, indicating that the crystal structure of Li2FeSiO4 has not been affected by Li3PO4. When the molar ratio of Li3PO4 is up to 6%, the star-labeled orthogonal phase Li3PO4 (JCPDS NO. 71-1528) appears in the samples. The diffraction peaks of Li3PO4 exhibit an increasing trend with enhanced amounts of Li3PO4. However, there are no characteristic peaks of Li3PO4 for LFS-4, which may be attributed to a small amount of introduced Li3PO4. There are also no diffraction peaks from the carbon phase shown in the patterns.
The morphologies of the samples with different contents of Li3PO4 were identified by SEM and the images are displayed in Fig. 4. From that, we can see the average size of nanoparticles is around 20 nm, and they are agglomerated together. The samples with different contents of Li3PO4 also preserve the morphology of Li2FeSiO4/C (Fig. 2b) with slightly decreased diameter, suggesting that Li3PO4 added has no effect on the morphology of Li2FeSiO4/C composite. To study the microstructure of LFS-6, TEM and HRTEM of material were characterized. TEM image (Fig. 5a) shows that the particles of LFS-6 are coated with amorphous carbon. From HRTEM image shown in Fig. 5b, we can clearly see the lattice fringes of Li2FeSiO4 and Li3PO4, respectively. Moreover, the interplanar spacing of 0.330、0.235 and 0.283 nm can be well assigned to the (201), (212) and (112) facets of monoclinic Li2FeSiO4, while the interplanar spacing of 0.231 nm correlated well with the (021) plane of orthogonal phase Li3PO4. The above results suggest that Li3PO4 and Li2FeSiO4/C form nanocomposite in LFS-6.
To investigate the influences of Li3PO4 content on the rate performance and high-rate cyclability of Li2FeSiO4/C, charge–discharge experiments were performed at voltage window of 1.5–4.8 V. Figure 6a exhibits the rate performance of LFS, LFS-4, LFS-6, LFS-8 and LFS-10 at different rates from 1 C to 10 C (1 C = 166 mA g−1) for 50 cycles each. It is apparently observed that Li2FeSiO4/C with different contents of Li3PO4 exhibit much better performance than LFS, except that LFS-10 is almost the same as that of LFS at 1 C and 2 C rates. Among the samples after adding fast ion conductor Li3PO4, LFS-6 possesses the best rate performance. This indicates that the introduction of Li3PO4 has a significant effect on improving the rate property of Li2FeSiO4/C, and the advantage is much more obvious on the high rate. Figure 6b presents the cycling performance of LFS, LFS-4, LFS-6, LFS-8 and LFS-10 at 10 C for 100 cycles. It is evident from the figure that the performance of LFS-6 is the best. The discharge capacity is 114.1 mAh g−1, and there is no capacity decay after 100 cycles. In addition, the cyclic stability of Li3PO4-Li2FeSiO4/C composites are higher than that of LFS without Li3PO4.
LFS and LFS-6 were selected to further study the electrochemical performance differences between pristine sample and sample with Li3PO4 additive at 0.1 C at voltage window of 1.5–4.8 V. Figure 7 compares the charge–discharge curves of the two electrodes for the initial 5 cycles. Obviously, the charge–discharge curves of LFS-6 are similar to the LFS, which suggests the intrinsic properties of Li2FeSiO4 are not affected by Li3PO4. For both composites, the initial charge curves show two voltage plateaus at around 3.3 V and 4.3 V, which should be related to the Fe2+/Fe3+ and Fe3+/Fe4+ redox couples, respectively. However, in the subsequent charge–discharge process, the charge curves are quite different from those of the first cycle, which is attributed to structural arrangement [42]. From Fig. 7a, it can be seen the discharge capacities of LFS are 209.3, 199.4, 193.1, 186.6 and 184.8 mAh g−1 for the initial 5 cycles, respectively, and the capacity retention is 88.3%, while the discharge specific capacities of LFS-6 cathode material (Fig. 7b) are 195.5, 183.2, 180, 6, 179.8 and 179 mAh−1, respectively, with a capacity retention of 91.8%. The LFS shows higher capacities than LFS-6 composite, however, the value of capacity retention is slightly lower than that of LFS-6. This is mainly due to the fact that Li3PO4 has no electrochemical activity. Although it has a negative effect on the specific capacity of Li2FeSiO4, it can enhance the cyclic stability of material to a certain degree.
In order to understand the influence of Li3PO4 additive on the rate performance and cyclic stability of Li2FeSiO4 more comprehensively, we test LFS and LFS-6 at high rate, and results are plotted in Fig. 8. For LFS-6 (Fig. 8c and d), the discharge capacities are 188.8, 172.8, 154.2 and 114.1 mAh g−1, respectively, at rates of 1 C, 2 C, 5 C and 10 C. With the increasing current rate, the discharge capacities of the composite decrease gradually. After 100 cycles, the capacities are 164.9, 153.1, 152.4 and 118.9 mAh g−1, respectively, with 87.3%, 88.6%, 98.8% and 104.2% of capacity attainable. In Fig. 8a and b, the discharge capacities of LFS are 177.5, 159.2, 133.0 and 89.0 mAh g−1, respectively, under the same rates. After 100 cycles, the capacities declined to 129.9, 123.5, 109.1 and 75.9 mAh g−1, respectively, and their corresponding capacity retentions are 73.2%, 77.6%, 82.0% and 85.3%. Additionally, our Li2FeSiO4 with 6 mmol% Li3PO4 added shows superior performances with an excellent cyclability and an outstanding rate performance than those of the previous reported Li2FeSiO4 summarized in Table 2.
Cyclic voltammetry (CV) was conducted to analyze the kinetic characteristics of LFS and LFS-6 composites in the second cycle, and the curves are presented in Fig. 9. From Fig. 9, it is clearly seen that the CV curves of LFS-6 are similar to that of LFS, demonstrating that Li3PO4 adding has no effect on the electrochemical behavior. All the electrodes exhibit a major redox couple of Fe2+/Fe3+ at a scan rate of 0.1 mV s−1 in the voltage range of 1.5–4.8 V. The anodic peak at 3.04 V in LFS corresponds to the oxidation of Fe2+ to Fe3+, and the cathodic peak at 2.45 V involving with the reduction of Fe3+ to Fe2+, with a potential interval of 0.59 V between these two redox peaks. Compared with LFS, LFS-6 show little narrower separations of the redox peaks (a potential interval of 0.42 V), suggesting that LFS-6 has better electrochemical kinetics.
EIS measurements of LFS and LFS-6 were carried out to further study the kinetic properties of the materials. Figure 10a illustrates the Nyquist plots of the two composites and the equivalent circuit is shown inset. All the EIS spectra are divided into three characteristic regions. The semicircle at the high to medium frequency region that is assigned to the charge-transfer resistance (Rct) through the solid-electrolyte interfaces, and an inclined line in the low frequency range which represents the lithium ion diffusion in the cathode material. The small intercepts by the semicircle on the real axis (Zre) in the high frequency which usually correspond to the ohmic resistance of the cell (Re), including the resistance of electrolyte, separator and electrodes. The diffusion coefficient of lithium ion (DLi+) can be obtained based on the relationship between Zre and the reciprocal square root of the frequency (ω−1/2) in the low frequency region, according to the equations mentioned [48]. From the impedance parameters summarized in Table 3, it is evident that the Rct value of LFS-6 (112.09 Ω) is much smaller than that of LFS (281.67 Ω) before cycling. After 100 cycles, the Rct value of LFS increased considerably in comparison with that of LFS-6. The Rct of LFS increased to 472.99 Ω, but the Rct of LFS-6 was 183.43 Ω. Meanwhile, the lithium ion diffusion coefficients (D +Li ) of LFS and LFS-6 before cycling can be calculated as 2.50 × 10−15 and 2.04 × 10−14 cm2 s−1, respectively. After 100 cycles, the DLi+ value of LFS-6 is still higher than that of LFS. The results above clearly imply that LFS-6 shows a smaller value of charge transfer resistance with a faster diffusion coefficient. Obviously, the Li3PO4 adding suppress the rising of charge transfer resistance upon cycling and enhance the kinetics of lithium-ion diffusion (Fig. 10).
4 Conclusion
In summary, we synthesized and characterized Li2FeSiO4/Li3PO4/C composites, and compared their electrochemical performance with pristine one as LIB cathodes. The Li2FeSiO4/Li3PO4/C composites exhibited superior rate capability and largely improved cycling performance, especially with the amount as high as 6 mmol%. The specific discharge capacities of LFS-6 can be achieved 188.8, 172.8, 154.2 and 114.1 mAh g−1 at 1 C, 2 C, 5 C and 10 C, respectively, and retained 164.9, 153.1, 152.4 and 118.9 mAh g−1 over 100 cycles. The overall improved electrochemical performances are mainly attributed to the improved lithium ion diffusion, promoted charge transfer process and retarded side reactions by introducing lithium ion conductor Li3PO4. Accordingly, adding Li3PO4 into Li2FeSiO4 can be a useful way to optimize the electrochemical performance, which can also be extended to other cathodes for LIBs.
References
M. Armand, J.M. Tarascon, Building better batteries. Nature 451, 652–657 (2008)
J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001)
G.E. Blomgren, The development and future of lithium ion batteries. J. Electrochem. Soc. 164(1), A5019–A5025 (2017)
J.B. Goodenough, K.-S. Park, The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135(4), 1167–1176 (2013)
D. Andre, S.-J. Kim, P. Lamp, S.F. Lux, F. Maglia, O. Paschos, B. Stiaszny, Future generations of cathode materials: an automotive industry perspective. J. Mater. Chem. A 3(13), 6709–6732 (2015)
J. Ni, Y. Jiang, X. Bi, L. Li, J. Lu, Lithium iron orthosilicate cathode: progress and perspectives. ACS Energy Lett. 2(8), 1771–1781 (2017)
H. Wei, X. Lu, H.-C. Chiu, B. Wei, R. Gauvin, Z. Arthur, V. Emond, D.-T. Jiang, K. Zaghib, G.P. Demopoulos, Ethylenediamine-enabled sustainable synthesis of mesoporous nanostructured Li2FeIISiO4 particles from Fe(III) aqueous solution for li-ion battery application. ACS Sustain. Chem. Eng. 6, 7458–7469 (2018)
R. Dominko, Li2MSiO4 (M = Fe and/or Mn) cathode materials. J. Power Sources 184, 462–468 (2008)
A. Kokalj, R. Dominko, G. Mali, A. Meden, M. Gaberscek, K. Jamnik, Beyond one-electron reaction in Li cathode materials: designing Li2MnxFe1−xSiO4. Chem. Mater. 19, 3633–3640 (2007)
A. Liicat, J. Thomas, Li-ion migration in Li2FeSiO4-related cathode materials: a DFT study. Solid State Ion. 192, 58–64 (2011)
L. Yi, X. Wang, G. Wang, Y. Bai, M. Liu, X. Wang, R. Yu, Improved electrochemical performance of spherical Li2FeSiO4/C cathode materials via Mn doping for lithium-ion batteries. Electrochim. Acta 222, 1354–1364 (2016)
A. Kumar, O.D. Jayakumar, Jagannath, P. Bashiri, G.A. Nazri, V.M. Naik, R. Naik, Mg doped Li2FeSiO4/C nanocomposites synthesized by the solvothermal method for lithium ion batteries. Dalton Trans. 46, 12908–12915 (2017)
H. Qiu, H. Yue, X. Wang, T. Zhang, M. Zhang, Z. Fang, X. Zhao, G. Chen, Y. Wei, C. Wang, D. Zhang, Titanium-doped Li2FeSiO4/C composite as the cathode material for lithium-ion batteries with excellent rate capability and long cycle life. J. Alloys Compd. 725, 860–868 (2017)
C. Deng, S. Zhang, S.Y. Yang, B.L. Fu, L. Ma, Synthesis and characterization of Li2Fe0.97M0.03SiO4 (M = Zn2+, Cu2+, Ni2+) cathode materials for lithium ion batteries. J. Power Sources 196, 386–392 (2011)
J. Yang, X. Kang, D. He, T. Peng, L. Hu, S. Mu, Hierarchical shuttle-like Li2FeSiO4 as a highly efficient cathode material for lithium-ion batteries. J. Power Sources 242, 171–178 (2013)
J. Yang, X. Kang, D. He, A. Zhang, M. Pan, S. Mu, Graphene activated 3D-hierarchical flower-like Li2FeSiO4 for high-performance lithium-ion batteries. J. Mater. Chem. A 3, 16567–16573 (2015)
X. Wu, X. Wang, Y. Zhang, Nanoworm like Li2FeSiO4-C composites as lithium-ion battery cathodes with superior high-rate capability. ACS Appl. Mater. Interfaces. 3, 2510–2516 (2013)
Y. Xu, W. Shen, A. Zhang, H. Liu, Z. Ma, Template-free hydrothermal synthesis of Li2FeSiO4 hollow spheres as cathode materials for lithium-ion batteries. J. Mater. Chem. A 2, 12982–12990 (2014)
Z. Ding, J. Liu, J. Ran, X. Zeng, S. Yang, A. Pan, D.G. Ivey, W. Wei, Three-dimensionally ordered macroporous Li2FeSiO4/C composite as a high performance cathode for advanced lithium ion batteries. J. Power Sources 329, 297–304 (2016)
Q. Zhang, C. Yan, Y. Meng, X. Wang, Hierarchical mesoporous Li2FeSiO4/C sheaf-rods as a high-performance lithium-ion battery cathode. J. Alloys Compd. 767, 195–203 (2018)
Y. Fujita, T. Hira, K. Shida, M. Tsushida, J. Liao, M. Matsuda, Microstructure of high battery-performance Li2FeSiO4/C composite powder synthesized by combing different carbon sources in spray-freezing/freeze-drying process. Ceram. Int. 44, 11211–11217 (2018)
S. Sun, C.M. Ghimbeu, C. Vis-Guterl, M.-T. Sougrati, C. Masquelier, R. Janot, Synthesis of Li2FeSiO4/carbon nano-composites by impregnation method. J. Power Sources 284, 574–581 (2015)
X. Huang, X. Li, H. Wang, Z. Pan, M. Qu, Z. Yu, Synthesis and electrochemical performance of Li2FeSiO4/carbon/carbon nano-tubes for lithium ion battery. Electrochim. Acta 55, 7362–7366 (2010)
K.C. Kam, T. Gustafsson, J.O. Thomas, Synthesis and electrochemical properties of nanostructured Li2FeSiO4/C cathode. Solid State Ion. 192, 356–359 (2011)
J. Bai, Z. Gong, D. Lv, Y. Li, H. Zou, Y. Yang, Nanostructured 0.8Li2FeSiO4/0.4Li2SiO3/C composite cathode material with enhanced electrochemical performance for lithium-ion batteries. J. Mater. Chem. 22, 12128–12132 (2012)
D. Li, R. Xie, M. Tian, S. Ma, L. Gou, X. Fan, Y. Shi, H.-T.-H. Yong, L. Hao, Improving high-rate performance of mesoporous Li2FeSiO4/Fe7SiO10/C nanocomposite cathode with a mixed valence Fe7SiO10 nanocrystal. J. Mater. Chem. A 2, 4375–4383 (2014)
H. Qiu, H. Yue, T. Zhang, T. Li, C. Wang, G. Chen, Y. Wei, D. Zhang, Enhanced electrochemical performance of Li2FeSiO4/C cathode materials by surface modification with AlPO4 nanosheets. Electrochim. Acta 222, 1870–1877 (2016)
K. Gaur, A.J. Pathak, H.B. Lal, Ionic and electronic conductivity in some simple lithium salts. J. Mater. Sci. 23, 4257–4262 (1988)
C. Lee, P.K. Dutta, R. Ramamoorthy, S.A. Akbar, Mixed ionic and electronic conduction in Li3PO4 electrolyte for a CO2 gas sensor. J. Electrochem. Soc. 153, H4–H14 (2005)
C.-H. Jo, D.-H. Cho, H.-J. Noh, H. Yashiro, Y.-K. Sun, S.T. Myung, An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res. 8, 1464–1479 (2015)
H.G. Song, J.Y. Kim, K.T. Kim, Y.J. Park, Enhanced electrochemical properties of Li(Ni0.4Co0.3Mn0.3)O2 cathode by surface modification using Li3PO4-based materials. J. Power Sources 196, 6847–6855 (2011)
Y. Jin, N. Li, C.H. Chen, S.Q. Wei, Electrochemical characterizations of commercial LiCoO2 powders with surface modified by Li3PO4 nanoparticles. Electrochem. Solid-State Lett. 9(6), A273–A276 (2006)
H. Konishi, K. Suzuki, S. Taminato, K. Kim, Y. Zheng, S. Kim, J. Lim, M. Hirayama, J.-Y. Son, Y. Cui, R. Kanno, Effect of surface Li3PO4 coating on LiNi0.5Mn1.5O4 epitaxial thin film electrodes synthesized by pulsed laser deposition. J. Power Sources 269(4), 293–298 (2014)
S.-X. Zhao, H. Ding, Y.-C. Wang, B.-H. Li, C.-W. Nan, Improving rate performance of LiFePO4 cathode materials by hybrid coating of nano-Li3PO4 and carbon. J. Alloys Compd. 566, 206–211 (2013)
X. Li, R. Yang, B. Cheng, Q. Hao, H. Xu, J. Yang, Y. Qian, Enhanced electrochemical properties of nano-Li3PO4 coated on the LiMn2O4 cathode material for lithium ion battery at 55°C. Mater. Lett. 66, 168–171 (2012)
Z. Wang, S. Luo, J. Ren, D. Wang, X. Qi, Enhanced electrochemical performance of Li-rich cathode Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by surface modification with lithium ion conductor Li3PO4. Appl. Surf. Sci. 370, 437–444 (2016)
X. Bian, Q. Fu, X. Bie, P. Yang, H. Qiu, Q. Pang, G. Chen, F. Du, Y. Wei, Improved electrochemical performance and thermal stability of Li-excess Li1.18Co0.15Ni0.15Mn0.52O2 cathode material by Li3PO4 surface coating. Electrochim. Acta 174, 875–884 (2015)
H. Liu, C. Chen, C. Du, X. He, G. Yin, B. Song, P. Zuo, X. Cheng, Y. Ma, Y. Gao, Lithium-rich Li1.2Ni0.13Co0.13Mn0.54O2 oxide coated by Li3PO4 and carbon nanocomposite layers as high performance cathode materials for lithium ion batteries. J. Mater. Chem. A 3, 2634–2641 (2015)
F. Wu, X. Zhang, T. Zhao, L. Li, M. Xie, R. Chen, Surface modification of cobalt-free layered Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 oxide with FePO4/Li3PO4 composite as the cathode for lithium-ion batteries. J. Mater. Chem. A 3, 9528–9537 (2015)
X. Wu, X. Jiang, Q. Huo, Y. Zhang, Facile synthesis of Li2FeSiO4/C composites with triblock copolymer P123 and their application as cathode materials for lithium ion batteries. Electrochim. Acta 80, 50–55 (2012)
S.I. Nishimura, S. Hayase, R. Kanno, M. Yashima, N. Nakayama, A. Yamada, Structure of Li2FeSiO4. J. Am. Chem. Soc. 130, 13212–13213 (2008)
A. Nyten, S. Kamali, L. Haggstrom, T. Gustafsson, J.O. Thomas, The lithium extraction/insertion mechanism in Li2FeSiO4. J. Mater. Chem. 16, 2266–2272 (2006)
H. Qiu, K. Zhu, H. Li, T. Li, T. Zhang, H. Yue, Y. Wei, F. Du, C. Wang, G. Chen, D. Zhang, Mesoporous Li2FeSiO4@ordered mesoporous carbon composites cathode material for lithium-ion batteries. Carbon 87, 365–373 (2015)
X. Wang, C. Qing, Q. Zhang, W. Fan, X. Huang, B. Yang, J. Cui, Facile synthesis and enhanced electrochemical performance of Li2FeSiO4/C/reduced graphene oxide nanocomposites. Electrochim. Acta 134, 371–376 (2014)
L.-L. Zhang, S. Duan, X.-L. Yang, G. Liang, Y.-H. Huang, X.-Z. Cao, J. Yang, M. Li, M.C. Croft, C. Lewis, Insight into cobalt-doping in Li2FeSiO4 cathode material for lithium-ion battery. J. Power Sources 274, 194–202 (2015)
L. Qu, D. Luo, S. Fang, Y. Liu, L. Yang, S. Hirano, C.-C. Yang, Mg-doped Li2FeSiO4/C as high-performance cathode material for lithium-ion battery. J. Power Sources 307, 69–76 (2016)
H. Qiu, H. Yue, T. Zhang, Y. Ju, Y. Zhang, Z. Guo, C. Wang, G. Chen, Y. Wei, D. Zhang, Enhanced electrochemical performance of Li2FeSiO4/C positive electrodes for lithium-ion batteries via yttrium doping. Electrochim. Acta 188, 636–644 (2016)
A.J. Bard, J.R. Faulkner, Electrochemical methods, 2nd edn. (Wiley, New York, 2001), p. 231
Acknowledgements
This investigation is supported by National Science Foundation of China (Grant No. 21271145), the National Science Foundation of Hubei Province (Grant No. 2015CFB537) and the Science and Technology Innovation Committee of Shenzhen Municipality (contract NO. JCYJ20170306171321438).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Y., Zan, L. & Zhang, Y. Effects of Li3PO4 additive on the electrochemical properties of Li2FeSiO4 as cathode material for lithium-ion batteries. J Mater Sci: Mater Electron 30, 15582–15591 (2019). https://doi.org/10.1007/s10854-019-01934-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01934-5