Abstract
In this paper, the water droplet experimental method was used to in situ study the electrochemical migration and related behaviors of Sn-based lead-free solder. Results showed that the electrochemical migration short-circuit time decreased with voltage increasing. Electrochemical migration was difficult to occur when the voltage was less than 1.5 V. However, when the voltage was above 5 V, metal deposits were generated in a short time and caused a bridge between two electrodes, which led to short circuit. As spacing increased, the short-circuit time prolonged. The three-dimensional pattern between voltage, spacing and short-circuit time had been constructed, which is beneficial to estimate the short-circuit time value under a combination of any voltage and spacing within a certain range. When changed the concentration of NaCl solution, it was founded that dendrites which surface covered much precipitates were formed at a low concentration. At medium concentration, there was no dendrites but only formed a large amount of precipitates. At a high concentration, the dendrites which surface with less precipitates were showed again. The formation mechanisms of dendrites under different NaCl solution concentrations were analyzed. In addition, the effects of different materials on electrochemical migration behavior were also studied, founding that electrochemical migration products of Sn, Sn0.7Cu and Sn3Ag were mainly Sn and Sn oxides. The electrochemical migration products of Sn9Zn contain much Zn element apart from Sn and Sn oxides. At a lower voltage, Sn9Zn solder had better electrochemical migration resistance than Sn, Sn0.7Cu and Sn3Ag, but this phenomenon was not obvious at a higher voltage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nearly half a century of development, advanced packaging technology is developing in a depth direction. The main idea of post-Moore’s Law is to pursue heterogeneous device/chip module integration with “function doubling” as the next profit growth point. Thus, “dimension reduction”, “multiple functions” and “micro-structure” are the mainstream packaging forms [1]. It promotes the development of electronic devices toward miniaturization, light weight, high precision and high reliability, which results in surface density of printed circuit boards increased [2], and the size and spacing of solder balls/bonding wires reduced. The solder balls/bonding wires spacing may only with a few micrometers, and the connected object may be just a few tens of micrometers of metal plating or a wire with a diameter of a few millimeters [3]. This will inevitably cause some damages to the reliability of solder joints/bonding wires, printed circuit boards and even electronic components. Coupled with the increasingly harsh service environment of electronic equipment, such as working in an extremely humid environment, the reliability problems caused by electrochemical effects can not be ignored [3, 4]. The electrochemical migration (ECM) is one of them.
Electrochemical migration is an electrochemical phenomenon, which means electronic components work under a certain temperature and humidity, the water droplets may formed due to the condensation of water vapor, and dust or impurities in air may also be mixed them to constitute an electrolyte environment. If there is a potential difference between electrodes, the electrochemical migration is easy to occur [5].
Voltage and spacing are all important factors that affecting electrochemical migration. In addition, chloride ion can be derived from human sweat gland liquid, skin, fingerprint, liquid metal salt solution and so on. Due to the development needs, electronic products have been or will be in service in an environment that cannot avoid chloride ions. Therefore, it is also necessary to explore the influence of chloride ion concentration on electrochemical migration. It is hoped that the study of reliability of electronic components caused by electrochemical migration can provide some data support and theoretical guidance for weakening or preventing the failure of electronic equipment.
2 Experimental procedure
2.1 Sample preparation
First, Sn as an example, a Sn solder block was pressed into a thickness of 0.2 mm by a tableting machine and cut it into a solder piece which has a length of 10 mm and a width of 3 mm. The solder piece then molded with resin [6], and one of the cross sections was ground to expose the solder base. Then the solder piece was used as an electrode.
Next, the solder piece was fixed on a ceramic substrate so that the spacing between two working faces was 0.5 mm, and the two ends of the electrodes were connected with the two ends of the power source by copper wires.
2.2 Experimental system construction
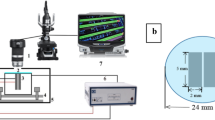
The water drop experiment system was built as shown in Fig. 1. Placing the prepared sample under a stereo microscope (Olympus SZX12), two copper wires respectively connected to both ends of the sample, and the other two ends connected to positive and negative poles of the power source. The end connected to positive pole was called anode, and the other end connected to negative pole was called cathode. In order to get a clear real-time video contrast image, Olympus LG-PS2 was used as a light source, then place a matching camera above stereo microscope and the camera was connected to computer, thus real-time observation and data recording can be realized on the computer screen.
For reliable in situ observation and experimental recording, the experimental setup was placed in a closed and clean room. Observed the formation of dendritic metal deposits under stereo microscope and recorded the entire process with a video camera. Nextly, the electrochemical migration short-circuit failure time was obtained by playing back the video [7]. SEM [Zeiss supra 55 (Vp)] was used to analyze the morphology of metal deposits, EDS and XRD to analyze the compositions and phases. Moreover, the electrochemical migration resistance was measured by electrochemical workstation.
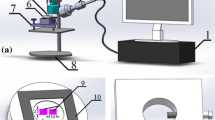
In order to measure the electrochemical migration resistance of different solders, the corrosion resistance of each solder was measured by a three-electrode electrochemical corrosion test, the experimental setup was shown in Fig. 2. A CS310 electrochemical workstation and a three-electrode system (anode is solder, cathode is the auxiliary electrode Pt, saturated calomel electrode is the reference electrode) were used to the experiment. The potential sweep range was − 1.5 to 2 V, and the scan voltage was 1 mV/s, the electrolyte was 3.5% NaCl solution. Next, the anodic polarization curve of each solder was measured by the potentiodynamic scanning method in a laboratory that the temperature is about 25 °C. So the electrochemical migration resistance of each solder was analyzed.
3 Results
3.1 Effect of voltage on ECM
3.1.1 Voltage influence on short-circuit time
Firstly, a concept should be introduced that short-circuit time refers to the time taken for metal deposits grow from cathode to anode and cause a bridge between two electrodes after electrochemical migration [7, 8].
Apply 1.5 V, 3 V, 5 V and 8 V to the pure Sn solder samples with an electrode spacing of 0.5 mm, each condition was repeated 7 times and averaged to obtain a voltage and short-circuit time chart as shown in Fig. 3. It can be seen that the short-circuit time drops sharply with voltage increasing. When the voltage was lower than 1.5 V, the short-circuit time was longer and the electrochemical migration phenomenon was difficult to occur. When the voltage was above 5 V, electrochemical migration occurred in a short time.
On the one hand, when applied voltage was higher, the anode dissolved and ion migration speed were faster. The Sn2+ from anode could reach cathode quickly and deposit under the action of electric field force and concentration gradient. On the other hand, the higher voltage, the more bubbles were produced between anode and cathode, and their strong agitation enhanced the mass transfer process [9], which promoted Sn2+ migrated to cathode in a short time and continuously formed metal deposits. In this way, the short-circuit failure time was short.
3.1.2 Effect of voltage on nucleation sites
When different voltages were applied to the pure Sn solder, the number of metal deposits, nucleation sites and growth ranges were various, as shown in Fig. 4. When the applied voltage was small (1.5 V), the metal deposits had fewer nucleation sites and a narrower growth range on the surface of cathode. When the applied voltage was large (5 V), the metal deposits had more nucleation sites and a wider growth range on cathode surface.
This was because the electric field strength at a small voltage was weak, the metal ions (Sn2+) dissolved in anode could migrate to cathode at a slow rate and had sufficient time to “find” its lowest energy point at cathode surface and then to deposit. The deposited atom formed a bump as the lowest energy point for next metal ion deposition. Thus, the anode was continuously dissolved, the cathode was continuously deposited, so there formed a small amount of metal deposits in a small range. At a large voltage, on the one hand, due to the large electric field strength, the dissolved metal ions of anode could quickly reach cathode, and it was too late to diffuse on the cathode surface to find its lowest energy point to deposit, so metal ions tend to randomly deposited. On the other hand, under a large voltage, the strong hydrogen evolution reaction at cathode could promote the random deposition of metal ions due to the agitation of bubbles, metal ions tended to nucleate randomly, so there were a large number of metal deposits and which had a wide growth range.
3.2 Influence of spacing on short-circuit time
When pure Sn was used as an electrode, we respectively applied different voltages (1.5 V, 3 V, 5 V and 8 V) at these samples with 0.3 mm, 0.5 mm and 0.7 mm spacing to measure the short-circuit time. Each condition was repeated 7 times and averaged the results. Finally, the results are shown in Fig. 5.
It can be seen from Fig. 5 that under a same voltage, the short-circuit failure time was gradually extended with spacing increase. However, this phenomenon was not obvious under 5 V or 8 V. Firstly, this may be caused by the larger spacing, the smaller electric field strength between electrodes, and the slower ion migration rate from anode to cathode. Secondly, the longer spacing between two electrodes and the longer distance required for metal deposits to grow from cathode to anode. Thus, a greater spacing lead to a longer short-circuit time [4]. At 5 and 8 V, the electric field force was large and plays a leading role in ion migration. So short-circuit time was short and the tendency of short-circuit time extended with spacing increasing was not obvious.
3.3 Short-circuit time under combination of voltage and spacing
Based on above research, the data between voltage, spacing and short-circuit time were compiled. Taking voltage as the x-axis, spacing as the y-axis, short-circuit time as the z-axis, a three-dimensional figure had been constructed, show as the Fig. 6. The significance is that short-circuit time value can be intuitively estimated at a combination of any spacing between 0.3 and 0.7 mm and any voltage between 1.5 and 8 V.
3.4 Effect of NaCl solution concentration on ECM
3.4.1 Metal deposits at various NaCl solution concentrations
When pure Sn was used as an electrode, under 2 V voltage and 0.7 mm electrode spacing, different concentrations of NaCl solution of 1 mM/L (low concentration), 30 mM/L (medium concentration) and 500 mM/L (high concentration) were used as electrolytes to observe the electrochemical migration phenomenon, the result was shown in Fig. 7. We founded that at lower concentration (1 mM/L), dendritic metal deposits which surface covered more precipitates were formed between two electrodes, as shown in a and a1 of Fig. 7. Under medium concentration (30 mM/L), no dendrites were formed and only a-wall-like precipitates which cracks were clearly after dehydration formed on the anode side, as shown in b and b1 of Fig. 7. Under high concentration (500 mM/L), there were dendrites and precipitates that formed on anode side, but the surface of dendrites with less precipitates, as shown in c and c1 of Fig. 7. Thus, the formation mechanism of dendrites at low and high concentration should be different. In this way, we have measured pH values at different concentrations.
3.4.2 pH under different NaCl solution concentrations
Added pH reagent into NaCl solution (1 mM/L, 30 mM/L, 500 mM/L) to adjust the color to pale yellow, as shown in Fig. 8, which means the solution was a neutral electrolyte. Then applied 2 V voltage to the sample which with 0.7 mm spacing for electrochemical migration water drop experiment.
After 5 s of electrochemical migration experiment, it was observed that as NaCl concentration increased, the purple color near cathode became deeper, which means more OH− produced and led to stronger alkalinity, as show in Fig. 9. Thus, the strong alkali environment should be the reason of different formation mechanisms of dendrites at low or high concentrations [10]. According to relevant literatures, it was founded that at low concentration (1 mM/L), the precipitation formed between anode and cathode was discontinuous, so there was no obvious hindrance to ion migration. The Sn2+ from anode can reach cathode smoothly under the action of electric force and then reduced, so dendrites will coexist with precipitates. At medium concentration (30 mM/L), the initial reaction rate of anode and cathode increased and the concentration of Sn2+ and OH− increased rapidly too, then they will form a large amount of precipitates when Sn2+ and OH− met each other, so there appeared a large precipitate layer. Sn2+ tries to migrate through precipitate layer to cathode and OH− tries to migrate through it to anode. However, each time they met and new precipitates formed, so a-wall-like precipitates appeared. At high concentration (500 mM/L), the pH of solution near cathode increased with chloride ion concentration increasing. When the concentration of chloride ion was more than 500 mM/L, the pH near cathode was close to 14 and the pH reagent appeared dark purple. Therefore, this strong alkali environment could dissolve the precipitate layer (hydroxide of Sn) and formed [Sn(OH)6]2− complex ions. It could reach cathode when pH ≥ 13 and be reduced to Sn [10, 11]. Therefore, dendrites formed, and the reaction equation as shown in Eq. (1).
3.5 Effect of different materials on ECM
3.5.1 Microscopic morphology of metal deposits
The sample with electrochemical migration products was placed in air to dry naturally. The sample was sprayed with carbon by ion sputter and deposits microscopic morphology was observed under SEM, the results were shown in Fig. 10. It was founded that deposits microstructures of Sn, Sn0.7Cu and Sn3Ag were similar, they all had many small branches grow on trunks and obviously dendritic. The morphology of Sn9Zn deposits was differently, which also had trunks, but the branches on trunks were relatively thick, and the whole was similar to feather [11].
3.5.2 Metal deposits composition
In order to investigate the reason of differences in microstructure of metal deposits, EDS and XRD analysis were carried out. Founding that the main components of Sn, Sn0.7Cu and Sn3Ag metal deposits were Sn and O elements. In addition to Sn and O elements, Sn9Zn metal deposits also contained a large amount of Zn element, as shown in Table 1, the C was from carbon-spray treatment, Al was from ceramic substrate, and "*" means inexistence of Zn atom in the corresponding solders.
Subsequently, XRD phase analysis revealed that the main phases of the Sn, Sn0.7Cu, and Sn3Ag metal deposits were Sn and oxides of Sn, Such as SnO2, SnO and Sn2O3 [12,13,14]. However, Sn9Zn metal deposits contained more Zn and ZnO in addition to Sn and oxides of Sn, as shown in Fig. 11, and the result was also consistent with the EDS results. Thus, the presence of Zn was responsible for the specialization of microstructure of Sn9Zn metal deposits [15, 16].
3.5.3 ECM easiness of different materials
Electrochemical migration is caused by anodic dissolution. Different materials have different corrosion potentials and the difficulty of electrochemical migration is different. It can be seen from the anodic polarization curves (shown in Fig. 12): dissolution potential: Sn9Zn < Sn < Sn0.7Cu < Sn3Ag, so electrochemical migration resistance was presumed: Sn9Zn < Sn < Sn0.7Cu < Sn3Ag.
In actual experiment, as shown in Fig. 13. The short-circuit failure time of Sn, Sn0.7Cu and Sn3Ag at 1.5 and 8 V were: tSn < tSn0.7Cu < tSn3Ag, this result indicated their electrochemical migration resistance: Sn < Sn0.7Cu < Sn3Ag, this was also consistent with the above inference. Compared to pure Sn, because Cu in Sn0.7Cu existed in the form of Cu6Sn5, and Ag in Sn3Ag existed in the form of Ag3Sn. However, Cu6Sn5 and Ag3Sn were both intermetallic compounds, and their corrosion resistance were extremely strong, so the electrochemical migration resistance of pure Sn was weaker than Sn0.7Cu and Sn3Ag. In addition, it was known from the composition ratio of Sn0.7Cu and Sn3Ag that the content of intermetallic compound (Ag3Sn) in Sn3Ag was much higher than the content of Cu6Sn5 in Sn0.7Cu, so the electrochemical migration resistance performance were: Sn0.7Cu < Sn3Ag. In summary, electrochemical migration resistance: Sn < Sn0.7Cu < Sn3Ag.
Sn9Zn had the lowest corrosion potential and was the easiest to electrochemical migration theoretically [17]. However, it was found in the experiment that as shown in Fig. 13, at a small voltage (1 V), the short-circuit failure time of Sn9Zn was the longest; At a large voltage (8 V). the short-circuit failure time of Sn9Zn was significantly reduced. Because it was well known that the surface of Sn9Zn was easy to form a passivation film in electrochemical experiments [18, 19], it had the effect of hindering the dissolution of anode. At a small voltage, the acts of Sn9Zn passivation film which could inhibit the anodic dissolution to a certain extent was obviously, thereby the passivation could further inhibit the occurrence of electrochemical migration [6], so the short-circuit failure time of Sn9Zn was longer at a lower voltage (1.5 V). However, the electric field strength at a large voltage was very strong, and the passivation film was broken or the passivation film formed was not so dense due to voltage breakdown or strong agitation of bubbles [20]. Therefore, the effect of Sn9Zn passivation film on inhibiting anodic dissolution was not significant, the electrochemical migration resistance was reduced, and the short-circuit failure time was reduced obviously.
As can be seen from the above: at a lower voltage (1.5 V), electrochemical migration resistance: Sn < Sn0.7Cu < Sn3Ag < Sn9Zn;At a large voltage (8 V), electrochemical migration resistance: Sn9Zn < Sn < Sn0.7Cu < Sn3Ag.
4 Conclusions
-
(1)
As voltage increased, electrochemical migration short-circuit time decreased. Below 1.5 V, electrochemical migration was difficult to occur. Above 5 V, metal deposits were formed in a short time and caused a bridge between two electrodes. What’s more, the short-circuit time was prolonged as spacing increased. And the three-dimensional graph between voltage, spacing, and short-circuit-time could be used to predict the short-circuit time value under any combination of voltage and spacing within a certain range.
-
(2)
At a small concentration of NaCl solution, dendrites which surface covered more precipitates were formed. At medium concentration, there were no dendrites and only a large amount of precipitates appeared. At high concentration, there were dendrites which surface with less precipitates. It was founded that the strong alkali environment at high concentration was responsible for the different dendrites formation mechanisms at low or high NaCl solution concentration.
-
(3)
The electrochemical migration products of Sn, Sn0.7Cu and Sn3Ag were mainly Sn and oxides of Sn. In addition to Sn and oxides of Sn, Sn9Zn electrochemical migration products had more Zn elements. And at a lower voltage (1 V), Sn9Zn solder had better electrochemical migration resistance than Sn, Sn0.7Cu and Sn3Ag. But this phenomenon was not so obvious at a higher voltage (8 V). Because Sn9Zn passivation film effect was remarkable at low voltage. However, at a high voltage, the effect will be weakened due to voltage breakdown or strong agitation of bubbles.
References
L. Long, Development trend of electronic packaging technology. Electron. Packag. 12, 39–41 (2012)
G. Aspar, B. Goubault, O. Lebaigue, et al. 3D printing as a new packaging approach for MEMS and electronic devices, Electron. Compon. Technol. Conf. (2017)
T. Tilford, S. Stoyanov, J. Braun, Design, manufacture and test for reliable 3D printed electronics packaging. Microelectron. Reliab. 85, 109–117 (2018)
C. Bailey, S. Stoyanov, T. Tilford, 3D-Printing and electronic packaging-current status and future challenges. Electron. Packag. Technol. Conf. 32, 850–853 (2017)
P. Yang, Y.L. Zhou, Experimental study on electrochemical migration of Ag impregnated circuit board. Electromec. Elem. 32, 43–47 (2012)
L. Hua, X.P. Guo, J.K. Yang, Electrochemical corrosion behavior and dendritic growth of Sn0.7Cu solder on FR-4 printed circuit board with Cu. Chin. J. corros. Prot. 30, 469–474 (2010)
H. Ma, A. Kunwar, J. Chen, Study of electrochemical migration based transport kinetics of metal ions in Sn-9Zn alloy. Microelectron. Reliab. 83, 198–205 (2018)
B. Liao, W. Jia, R. Sun, Electrochemical migrition behavior of Sn–3.0Ag–0.5Cu solder alloy under thin electrolyte layers. Surf. Rev. Lett. 208, 1–7 (2018)
W. Zhang, D.H. Cheng, Z.Z. Yu, W. Jillek, In situ observation and research on electrochemical Migration of Sn–Ag–Cu solder joints. Electron. Compon. Mater. 26, 64–68 (2007)
X. Zhong, L. Chen, B. Medgyes, Electrochemical migration of Sn and Sn solder alloys: a review. RSC Adv. 7, 28186–28206 (2017)
X.K. Zhong, The Corrosion and Electrochemical Migration of Tin under Thin Electrolyte Layers (Huazhong University of Science and Technology, Wuhan, 2014)
Y. Zhong, A.P. Xian, Corrosion of Ga-doped Sn-0.7Cu Solder in simulated marine atmosphere. Metal. Mater. Trans. A 44, 1462–1474 (2013)
F. Rosalbino, E. Angelini, G. Zanicchi, Electrochemical corrosion study of Sn–3Ag–3Cu solder alloy in NaCl solution. Electrochim. Acta 54, 7231–7235 (2009)
D.H. Nam, R.H. Kim, W.H. Dong, Electrochemical performances of Sn anode electrodeposited on porous Cu foam for Li-ion batteries. Electrochim. Acta 66, 126–132 (2012)
W. Zhang, In-situ Observation and Study on Electrochemical Migration of Lead-free Solders (Shanghai University, Shanghai, 2007)
G.M. Mena, Analysis of dendrite images formed by electrochemical migration in a semiconductor sensor. Mater. Sci. Forum. 916, 207–211 (2018)
Z.G. Fan, H.T. Ma, L. Wang, Effect of Cu on electrochemical corrosion properties of Sn-9Zn lead-free solder. Chin. J. Nonferrous Met. 17, 1302–1306 (2007)
Z.A. Mohd Rasid, M.H. Zainol, M.F. Omar, Corrosion performance of Sn-9Zn and Sn-0.7Cuin 3.5% NaCl solution. Solid State Phenom. 273, 56–60 (2018)
Z. Wang, C. Chen, J. Jiu, Electrochemical behavior of Sn-9Zn-x Ti lead-free solders in neutral 0.5 M NaCl solution. J. Mater. Eng. Perform. 27, 2182–2191 (2018)
A. Ahmido, A. Sabbar, H. Zouihri, Effect of bismuth and silver on the corrosion behavior of Sn–9Zn alloy in NaCl 3wt% solution. Mater. Sci. Eng. B 176, 1032–1036 (2011)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos: 51871040 and 51571049).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, X., Ma, H., Wang, C. et al. Electrochemical migration behavior of Sn-based lead-free solder. J Mater Sci: Mater Electron 30, 14695–14702 (2019). https://doi.org/10.1007/s10854-019-01841-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01841-9