Abstract
The electrical performance of solution-processed, small-molecule organic semiconductors is largely restricted by their severe charge carrier mobility variations. In this work, we demonstrate an effective method to reduce such variations of the semiconductor mobilities and improve the performance consistency of organic thin film transistors (OTFTs) by adding poly(α-methylstyrene) (PαMS) as a polymer additive. By using 6,13-bis(triisopropylsilylethynyl) pentacene (TIPS pentacene) as an example, we found that while pristine TIPS pentacene film exhibited random crystal orientation and large film gaps, the addition of PαMS polymer promoted the growth of semiconductor crystals, which formed uniformly aligned needles with significantly improved orientation and coverage within the channel regions. An ultra-low misorientation angle of 2.2° ± 1° was obtained from TIPS pentacene/PαMS blend film, which is a 20-fold reduction as compared to pristine TIPS pentacene. Bottom-gate, top-contact OTFTs with TIPS pentacene crystals aligned perpendicularly from source to drain contact electrodes demonstrated a hole mobility of up to 0.26 cm2/V s, as well a 6-fold enhancement of average mobility as compared to the pristine TIPS pentacene based counterparts. Notably, the addition of PαMS led to a performance consistency factor of 3.35, as defined by the ratio of the average mobility to standard deviation, implying a great reduction of charge carrier mobility variations. The improvement of electrical performance of OTFTs can be attributed to the combined outcome of crystal rigid alignment, extended long-range order, and almost full coverage of charge transport channel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The study of solution-processed small-molecule organic semiconductors has attracted great attention in recent years, unlocking potential applications in high-performance organic electronics devices on large-scale, low-cost flexible substrate [1,2,3,4,5,6,7,8]. Despite much progress achieved in the study of their charge carrier mobility [9,10,11,12,13], the random crystallization of these organic semiconductors still remains as a major problem. For example, the small-molecule organic crystals from drop casting in solution tended to grow in random directions, resulting in a film with crystal misorientation and extremely low coverage on substrate [14, 15]. The charge transport based on this type of morphology exhibits significant variations as the mobility measured from device to device would greatly vary [16, 17].
In order to address these aforementioned problems, various external alignment methods have been applied by different groups to physically control the crystal growth of the small-molecule organic semiconductors. For example, Asare-Yeboah et al. reported a temperature gradient technique to control the crystal orientation of a p-type small-molecule organic semiconductor 6,13-bis(triisopropylsilylethynyl) pentacene (TIPS pentacene). The application of the temperature gradient technique tuned the solute solubility and created a higher nucleation seed density at the lower temperature side due to supersaturation, from which TIPS pentacene crystals were promoted to grow oriented towards the higher temperature side [18]. Bi et al. reported a “controlled evaporative self-assembly (CESA)” method, which combined a capillary force alignment setup and a double-solvent scheme, to grow a (2,5-di-(2-ethylhexyl)-3,6-bis(5″-n-hexyl-2,2′,5′,2″]terthiophen-5-yl)-pyrrolo [3,4-c]pyrrole-1,4-dione) (SMDPPEH) semiconductor with aligned crystal orientation and enhanced film coverage [19]. The use of double solvent led to the formation of crystal nucleation seeds, from which the SMDPPEH crystals grew into well-aligned, elongated needles, as aligned by the capillary force.
Apart from the external alignment methods, polymer additives have been demonstrated as a useful tool to tune the crystal growth and charge transport of the small-molecule organic semiconductors. The semiconductor/polymer blends benefit from the following aspects. First, the blend system can take advantage of both the high charge transport from the semiconductor and uniformity property from the polymer [20]. Second, certain polymer additives were known to induce a vertical phase segregation between the polymer and semiconductor, providing a three-dimensional confinement of the semiconductor charge transport [21, 22]. For example, a series of the polyacrylate polymers were reported to blend with TIPS pentacene. The resultant semiconductor/polymer blend system exhibited different forms of phase segregation, which ranged from lateral phase segregation, to vertical segregation, and to a combination of both, dependent upon the length of the alkyl side group of polyacrylate polymer additive [23]. In addition, polystyrene (PS) was reported to be blended as a polymer additive with small-molecule organic semiconductors [24,25,26,27,28,29]. Particularly, a vertical phase segregation was found to be existent from a spin-coated TIPS pentacene/PS blends [30]. Third, conjugated polymers were reported to provide a unique pathway to modify the polymorphism and thin film morphology of semiconductors via the intermolecular interactions between the polymer and semiconductor. Chen et al. for the first time studied the influence of different extent of intermolecular interactions on the charge transport of semiconductors [20]. By blending TIPS pentacene with two different conjugated polymers, i.e. poly(3-hexylthiophene) (P3HT) and regiorandom pentacene-bithiophene (PnBT-RRa), it was found that PnBT-RRa provided a stronger intermolecular interaction resulted from both π–π and hydrophobic interactions, and correspondingly provided a more effective crystallization confinement and facilitated the semiconductor to form oriented needles with long-range order and rigid uniformity.
In this work, we reported the addition of poly(α-methylstyrene) (PαMS) as a polymer additive to control the crystal growth and modify the charge transport of small-molecule organic semiconductors. With TIPS pentacene studied as an example, we showcased that while the pristine TIPS pentacene crystals exhibited random crystal orientation and very low film coverage, the addition of the PαMS polymer resulted in a drastic 20-fold reduction of misorientation angle to 2.2° ± 1°, indicative of a great enhancement of crystal orientation. In addition, the addition of PαMS polymer led to an almost full coverage within the charge transport channel of organic thin film transistors (OTFTs). As a result, the TIPS pentacene/PαMS blend based OTFTs with a bottom-gate, top-gate configuration showed a mobility of up to 0.26 cm2/V s, as well as a 6-fold enhancement of average mobility as compared to the pristine TIPS pentacene based counterparts. Notably, the addition of PαMS polymer led to an enhanced performance consistency factor of 3.35. The improved electrical performance of the TIPS pentacene/PαMS OTFTs was mainly attributed to the rigid crystal alignment with extended long-range order and full coverage within the charge transport channel.
2 Experimental section
TIPS pentacene, PαMS and toluene were purchased from Sigma Aldrich. To grow the crystals, TIPS pentacene and PαMS were separately dissolved in the solvent toluene with a concentration of 5 mg/ml, before they were mixed in solution at a volume ratio of 1:1. Prior to the deposition of semiconductor materials, the substrates were performed with hexamethyldisilazane (HMDS) surfactant treatment by heating up the HMDS solution up to a temperature of 140 °C, keeping the substrate in an environment rich of HMDS vapors and then rinsing with IPA solution.
Approximately 300 μl of TIPS pentacene/PαMS mixture solution was drop casted onto a heavily-doping n-type silicon substrate which has a 250 nm thickness of thermally-grown silicon dioxide layer as gate dielectric. Two types of substrates were studied in this work, including with and without HMDS treatment prior to the deposition of the semiconductor materials. The substrate was placed inside a Petri dish and covered with a cap, which enabled a solvent rich environment. A small angle of tilting was added to the substrate to facilitate the crystals to grow along the specific direction. A typical crystallization process usually takes 0.5–1 h.
After the crystallization of the semiconductor TIPS pentacene materials was completed, OTFTs with a bottom-gate, top-contact configuration were fabricated to measure charge transport. In particular, gold (Au) was deposited through a shadow mask with an Angstrom Engineering thermal evaporator as source and drain contact electrodes. The deposition rate of gold was 1 Å/s at a pressure of 10−7 Torr. A large channel size was used in this work in order to showcase the extended orderliness of the crystals. The channel width as defined by the shadow mask was 2000 μm, whereas the channel length was from 25 to 100 μm. I-V measurement of OTFTs, including both output and transfer characteristics, was conducted using a Keithley 4200 model semiconductor parameter analyzer in an ambient environment. All OTFTs were measured for five time to ensure consistency. Optical images were taken by using a polarized optical microscope.
3 Results and discussion
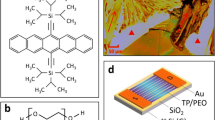
TIPS pentacene was used an example to generally represent solution-processed, small-molecule organic semiconductors, primarily due to the following semiconductor and materials properties of TIPS pentacene. As shown in its molecular structure of Fig. 1a, TIPS pentacene is a p-type small-molecule semiconductor with high mobility, thanks to its π–π stacking; in addition, the attachment of bulky side groups results in a disruption of the herringbone packing motif, leading to improved solvent solubility [14, 31,32,33,34,35]. The molecular structure of PαMS and toluene are shown in Fig. 1b and c, respectively.
When TIPS pentacene was drop casted in toluene, the semiconductor grew into randomly-oriented crystals, which were shown in the optical images of Fig. 2a, b. The charge transport based on such misoriented crystal needles is essentially anisotropic, which is indicated by the various directions of the long axis [210] or charge transport directions as represented by the red arrows. Apart from the crystal misorientation, the TIPS pentacene film also had poor coverage on the substrate, indicated by the white triangles. The measured hole mobility based on such thin film morphology exhibited a significant variation, leading to poor performance consistency of the TIPS pentacene based OTFTs [36]. In contrast, when PαMS was added to tune the thin film morphology of TIPS pentacene, the polymer additive facilitated the semiconductor to grow into elongated needles that extended in long-range order across the substrate. As indicated by the red arrow directions of Fig. 2c, d, the charge transport based on this morphology became more uniform as compared to pristine TIPS pentacene. In addition, the polymer additive results in almost a full coverage of TIPS pentacene on the substrate.
Polarized optical microscopy images of a–b pristine TIPS pentacene film and c–d TIPS pentacene/PαMS blend film with a 1:1 weight ratio. The red arrows indicate the directions of long axis [210] or charge transport directions of TIPS pentacene crystals. In a–b the tilting rods within the crystals represent the backbone direction, while the white triangles mark the bare substrate surface SiO2 without the coverage of TIPS pentacene crystals. All four images have the same scale bar of 50 μm as shown in d (Color figure online)
As can be seen from the optical images of Fig. 2, the addition of PαMS polymer additive has effectively improved the crystal alignment and tuned the crystal size. To more accurately measure such changes in the crystal orientation and size, we employed “grain width” and “misorientation angle”. The grain width of a TIPS pentacene crystal is measured along its short axis \([1\bar{2}0]\) as shown in the inset of Fig. 3b. Based on the calculation of 15 crystals, the TIPS pentacene crystals exhibited an average grain width of 38.9 ± 16.3 μm. As a comparison, the addition of PαMS polymer led to an average grain width of 25.7 ± 5.4 μm, as shown in the plot of Fig. 3a. Since the crystalline defects and trap centers are located at the grain boundaries of the TIPS pentacene crystals [37], reduced grain width would essentially introduce more defects and negatively impact charge transport of TIPS pentacene crystal based OTFTs.
a Grain width (WG) and b misorientation angle measurement of TIPS pentacene crystals, with and without PαMS polymer additive. The average and standard deviation results were both based on the measurement of 15 TIPS pentacene crystals. As indicated in by the inset of b, a TIPS pentacene crystal has [210] long axis, which is represented by the red arrow and indicates charge transport direction, as well as a \([1\bar{2}0]\) short axis. The grain width in a is calculated as the crystal width along the short axis. The misorientation angle in b is calculated as the angle between a crystal and another crystal which was picked as a baseline (Color figure online)
In addition, we use the concept of misorientation angle to demonstrate improvement of crystal random orientation. The misorientation angle of the crystals is calculated by using the angle between a particular crystal and another crystal that is picked as a measurement baseline. As can be seen from the optical images, the pristine TIPS pentacene exhibited severe random crystal misorientation, which led to a large misorientation angle of 43.9° ± 27.8° based on 15 measurement of TIPS pentacene crystals from Fig. 2a, b. The addition of PαMS polymer reduced the misorientation angle to an ultra-low value of 2.2° ± 1°, also based on 15 crystals from Fig. 2c, d. Impressively, it can be inferred that the addition of the PαMS polymer led to a 20-fold reduction of the misorientation angle.
To test the charge transport, bottom-gate, top-contact OTFTs were fabricated based on TIPS pentacene/PαMS blends. Two types of OTFTs were tested, with and without HMDS treatment. Figure 4 shows the optical images of the different devices from the same substrate with TIPS pentacene/PαMS blend, which showed that the crystal alignment was achieved for each OTFT and extended in long range–range order. Apart from the improved alignment, the addition of the PαMS polymer additive has greatly increased the areal coverage within the semiconductor channel, where charge transport takes place. A fully covered channel with crystals aligned perpendicularly from source to drain contact electrode is beneficial for the charge transport and device performance of TIPS pentacene based OTFTs. The measured mobility largely depends on the angle (θ) between the crystal orientation (also the long axis [210]) and the direction from source to drain contact electrodes. In particular, the measured mobility can attain values 10 times higher when the crystal orientation and the source to drain direction are in parallel (θ = 0°) than when they are perpendicular to each other (θ = 90°), according to a previous study [38]. Therefore, it is ideal to align the orientation of bulk TIPS pentacene crystals to be uniformly in parallel with the direction from source to drain electrodes, in order to maximize the charge transport.
a–d Optical images of different OTFTs from a substrate based on TIPS pentacene/PαMS blends, showing an almost crystal coverage in the charge transport channel and rigid crystal alignment that extended in long-range order for multiple devices across the same substrate. The regions highlighted in the red, dotted line in a refer to the source (S) and drain (D) contact electrodes. All images share the same scale bar of 50 μm as indicated in d (Color figure online)
The representative output characteristics and transfer characteristics of TIPS pentacene/PαMS based OTFTs were shown in Fig. 5. In particular, Fig. 5a, b were based on devices without HMDS treatment on the SiO2 gate dielectrics, whereas Fig. 5a, b were based on devices with HMDS treatment. From the transfer characteristics, the field-effect mobilities of OTFTs were extracted based on the following Eq. (1):
where μ means the OTFT field-effect mobility, Ci means the capacitance of gate dielectrics (~ 13.8 nF/cm2 for SiO2 in this work); W and L means the width and length of the semiconducting channel, respectively, which were defined by the dimension of the shadow mask, VT means the threshold voltage.
Figure 6a shows an illustration of the device structure of OTFTs with a bottom-gate, top-contact configuration. The average mobilities of TIPS pentacene/PαMS blend based OTFTs with and without HMDS treatment were compared with the mobilities of the devices based on pristine TIPS pentacene in Fig. 6b. While TIPS pentacene based OTFTs exhibited an average mobility of 0.03 ± 0.03 cm2/V s [36], the addition of PαMS polymer additive led to an average mobility of 0.071 ± 0.027 cm2/V s and 0.185 ± 0.055 cm2/V s, which is based on with and without HMDS treatment. In particular, the devices without HMDS treatment demonstrated a 6-fold enhancement of average mobility, which we contributed to the combined outcome of rigid crystal alignment, full charge transport channel coverage, film uniformity and extended long-range order, due to the addition of PαMS polymer additive. The lower average mobilities from the devices with HMDS treatment can be attributed to the fact that the HMDS surfactant treatment increased the hydrophobicity of the gate dielectrics, which caused a lower film coverage of TIPS pentacene in the channel and contributed to lower mobilities.
a A schematic of the bottom-gate, top-contact OTFTs used in this work, where “S” and “D” refers to the “source” and “drain” contact electrodes, respectively. b Average mobilities of OTFTs based on pristine TIPS pentacene crystals, TIPS pentacene/PαMS blends with and without HMDS treatment. c Variation of measured mobility of TIPS pentacene/PαMS blends with and without HMDS treatment on the SiO2 gate dielectrics, showing mobilities varying within one order of magnitude. d Performance consistency factor [calculated by the ratio of average mobility (μAVE) to standard deviation (μSTDEV)] of OTFTs. Both average and standard deviation mobility are based on the measurement of six OTFTs on each type of substrate. “TP” refers to TIPS pentacene in a–d
Figure 6c compares the variation of measured mobilities from device to device. The pristine TIPS pentacene based OTFTs showed mobilities that varied from 8.4 × 10−2 to 9.8 × 10−5 cm2/V s, indicating poor consistency of TIPS pentacene OTFTs [36]. The addition of the PαMS polymer has led to mobilities of 0.12–0.26 cm2/V s based on OTFTs without HMDS treatment, and mobilities of 0.52–0.11 cm2/V s based on OTFTs with HMDS treatment. Notably, while the pristine TIPS pentacene OTFTs exhibited mobilities that ranged by three orders of magnitude, the addition of the polymer has reduced such significant variation to within one order of magnitude for OTFTs both with and without HMDS treatment.
To investigate how the reduced mobility variation impacted the device performance consistency, we calculated the ratio of average mobility to standard deviation of mobility (μAVE/μSTDEV) and used this factor as a metric to evaluate the change of performance consistency. Without the addition of the polymer additive, the pristine TIPS pentacene based OTFTs exhibited a μAVE/μSTDEV factor of 1. In contrast, the TIPS pentacene/PαMS OTFTs showed an improved μAVE/μSTDEV factor of 2.69 and 3.35, respectively, which is based on with and without HMDS treatment. Impressively, the addition of polymer led to a 3-fold enhancement of performance consistency for OTFTs without HMDS treatment. It can be inferred that the reduced mobility variation can effectively enhance the performance consistency of TIPS pentacene crystal based OTFTs.
We use a schematic to illustrate how the addition of PαMS polymer additive affected the crystal growth, thin film morphology, charge transport and device performance of TIPS pentacene. As shown in Fig. 7a, the pristine TIPS pentacene has randomly-oriented crystals with a low areal coverage on the substrate, leading to significant measured mobility variations from OTFT to OTFT. The addition of the PαMS polymer has effectively addressed these issues from the following aspects. First, blending PαMS has improved the orientation of TIPS pentacene crystals, leading to the formation of elongated crystal needles with long-range order. Second, thanks to the uniformity property of the PαMS polymer, the TIPS pentacene/PαMS blend film exhibits almost a full coverage on the substrate, which was a tremendous enhancement as compared to inferior areal coverage of pristine TIPS pentacene crystals, as shown in the schematic of Fig. 7b. Third, blending TIPS pentacene with PαMS contributes to a vertical phase segregation between the semiconductor and the polymer, forming a trilayer structure with TIPS pentacene/PαMS/TIPS pentacene rich section from top to bottom of the active layer [21]. A thin layer with elevated concentration of TIPS pentacene was facilitated to form near the semiconductor/substrate interface where charge transport takes place, which is beneficial for charge transport and device performance of OTFTs [22].
A schematic illustrating a TIPS pentacene crystals with random orientation and poor coverage on substrate, and b TIPS pentacene/PαMS polymer blend crystals rigidly aligned in long-range order with increased substrate coverage. The red arrows in a and b refer to the direction of crystal long axis [210] of TIPS pentacene. The tilted rods in blue refer to the orientation of TIPS pentacene backbones (Color figure online)
The addition of PαMS polymer is expected to improve the stability of the TIPS pentacene based OTFTs. The effect of PαMS additive on the OTFT stability has been studied in a few previous reports. For example, Kim et al. reported improved operational stability of 2,8-difluoro-5,11-bis(triethylsilylethynyl) anthradithiophene (diF-TESADT)/PαMS based OTFTs [39], which was attributed to the block of oxygen and moisture from the ambient environment. Kang et al. reported improved stability of OTFTs with TIPS pentacene/PαMS thin film as an active layer, which was accredited to the formation of a polymeric encapsulation layer of the semiconductor channel [22]. Also, in our previous work, enhanced air stability was observed for N,N′-1H,1H-perfluorobutyl dicyanoperylenecarboxydiimide (PDIF-CN2) based OTFTs with PαMS as a polymer additive [40]. Such stability improvement was attributed to the hydrophobicity nature of the PαMS polymer, which effectively decreased the adsorption of moisture and thereby reduced the degradation of the semiconducting channel of PDIF-CN2. Therefore, we expect the addition of PαMS polymer as an additive to enhance the air stability of the TIPS pentacene based OTFTs.
A comparison of this work and selected references from Sect. 3 was presented in Table 1.
Finally, we would like to point out that the measured mobility of OTFTs was dependent on a few factors, including the surfactant treatment prior to the deposition of semiconductor layer, the application of external alignment methods, the dimension of charge transport channel, and etc. The OTFTs used in this work is based on a bottom-gate, top-contact configuration, in which case the gold contact electrodes were only deposited after the formation of the semiconductor crystals. As a result, it is not feasible to perform a pentafluorobenzene thiol (PFBT) surfactant treatment on the electrodes without damaging the TIPS pentacene layer, which was used to tune the work function of gold and facilitate transport of charge carriers by previous studies [41,42,43]. In addition, a majority of previous works used the actual dimension of the channel with crystal coverage as the channel width and length [44,45,46]. In contrast, we purposefully used a relatively large channel (width of 2000 μm and length of 25–100 μm), which was defined by the shadow mask, in order to demonstrate the extended long-range alignment of the TIPS pentacene crystals due to the addition of the polymer additive. A large channel dimension may cause an impact on the charge transport and lead to reduced mobilities because of the incorporation of more grain boundaries, where crystal defects and trap centers were typically existent [47]. Therefore, the mobility values reported in this work can be further increased if external alignment methods and PFBT surfactant treatment become available based on a different bottom-gate, bottom-contact device configuration.
4 Conclusions
In summary, we have reported a simple method to control the crystal growth, thin film morphology, charge carrier transport and performance consistency of TIPS pentacene small-molecule based OTFTs by blending TIPS pentacene with a PαMS polymer additive. The addition of PαMS facilitated the TIPS pentacene crystals to attain rigid alignment in a uniform direction, yielding an ultra-low misorientation angle of 2.2° ± 1°. Bottom-gate, top-contact OTFTs based on the TIPS pentacene/PαMS polymer additive blends exhibited a hole mobility of up to 0.26 cm2/V s and a μAVE/μSTDEV factor of 3.35, which is a higher than 3-fold enhancement of performance consistency compared to that of the TIPS pentacene based counterparts. Our method to tune the thin film morphology of small-molecule semiconductor and its device performance can find applications in low-cost, high-throughput organic electronics devices on a large-scale, flexible substrate.
References
S. Bi, Y. Li, Z.R. He, Z.L. Ouyang, Q.L. Guo, C.M. Jiang, Self-assembly diketopyrrolopyrrole-based materials and polymer blend with enhanced crystal alignment and property for organic field-effect transistors. Org. Electron. 65, 96–99 (2019)
J. Kwon, Y. Takeda, R. Shiwaku, S. Tokito, K. Cho, S. Jung, Three-dimensional monolithic integration in flexible printed organic transistors. Nat. Commun. (2019). https://doi.org/10.1038/s41467-018-07904-5
S. Bi, Q. Li, Y. Yan, K. Asare-Yeboah, T. Ma, C. Tang, Z. Ouyang, Z. He, Y. Liu, C. Jiang, Layer-dependent anisotropic frictional behavior in two-dimensional monolayer hybrid perovskite/ITO layered heterojunctions. Phys. Chem. Chem. Phys. 21, 2540–2546 (2019)
U. Jeong, G. Tarsoly, J. Lee, Y. Eun, J. Do, S. Pyo, Interdigitated ambipolar active layer for organic phototransistor with balanced charge transport. Adv. Electron. Mater. (2019). https://doi.org/10.1002/aelm.201800652
H. Li, Z. He, Z. Ouyang, S. Palchoudhury, C.W. Ingram, I.I. Harruna, D. Li, Modifying electrical and magnetic properties of single-walled carbon nanotubes by decorating with iron oxide nanoparticles. J. Nanosci. Nanotechnol. (2020). https://doi.org/10.1166/jnn.2020.17215
Z.R. Wang, Y. Zou, W.Q. Chen, Y.J. Huang, C.J. Yao, Q.C. Zhang, The role of weak molecular dopants in enhancing the performance of solution-processed organic field-effect transistors. Adv. Electron. Mater. (2019). https://doi.org/10.1002/aelm.201800547
Z.R. He, N. Lopez, X.L. Chi, D.W. Li, Solution-based 5,6,11,12-tetrachlorotetracene crystal growth for high-performance organic thin film transistors. Org. Electron. 22, 191–196 (2015)
M. Chu, J.X. Fan, S.J. Yang, D. Liu, C.F. Ng, H.L. Dong, A.M. Ren, Q. Miao, Halogenated tetraazapentacenes with electron mobility as high as 27.8 cm2 V−1 s−1 in solution-processed n-channel organic thin-film transistors. Adv. Mater. (2018). https://doi.org/10.1002/adma.201803467
C.Y. Wong, B.L. Cotts, H. Wu, N.S. Ginsberg, Exciton dynamics reveal aggregates with intermolecular order at hidden interfaces in solution-cast organic semiconducting films. Nat. Commun. 6, 7 (2015)
Z. He, J. Chen, D. Li, Review Article: crystal alignment for high performance organic electronics devices. J. Vac. Sci. Technol. A 37, 040801 (2019)
X.Y. Zheng, H. Geng, Y.P. Yi, Q.K. Li, Y.Q. Jiang, D. Wang, Z.G. Shuai, Understanding lattice strain-controlled charge transport in organic semiconductors: a computational study. Adv. Funct. Mater. 24, 5531–5540 (2014)
B.Y. Peng, S.Y. Huang, Z.W. Zhou, P.K.L. Chan, Solution-processed monolayer organic crystals for high-performance field-effect transistors and ultrasensitive gas sensors. Adv. Funct. Mater. 27, 1700999 (2017)
C.T. da Rocha, K. Haase, Y.C. Zheng, M. Loffler, M. Hambsch, S.C.B. Mannsfeld, Solution coating of small molecule/polymer blends enabling ultralow voltage and high-mobility organic transistors. Adv. Electron. Mater. 4, 1800141 (2018)
K. Asare-Yeboah, R.M. Frazier, G. Szulczewski, D. Li, Temperature gradient approach to grow large, preferentially oriented 6,13-bis(triisopropylsilylethynyl) pentacene crystals for organic thin film transistors. J. Vac. Sci. Technol. B 32, 052401 (2014)
Z. He, K. Asare-Yeboah, Z. Zhang, S. Bi, Self-assembly crystal microribbons with nucleation additive for high-performance organic thin film transistors. Jpn. J. Appl. Phys. (2019). https://doi.org/10.7567/1347-4065/ab1bae
Z.R. He, K. Xiao, W. Durant, D.K. Hensley, J.E. Anthony, K.L. Hong, S.M. Kilbey, J.H. Chen, D.W. Li, Enhanced performance consistency in nanoparticle/TIPS pentacene-based organic thin film transistors. Adv. Funct. Mater. 21, 3617–3623 (2011)
Z. He, Z. Zhang, S. Bi, Polyacrylate polymer assisted crystallization: improved charge transport and performance consistency for solution-processable small-molecule semiconductor based organic thin film transistors. J. Sci. Adv. Mater. Devices (2019). https://doi.org/10.1016/j.jsamd.2019.02.004
K. Asare-Yeboah, S. Bi, Z.R. He, D.W. Li, Temperature gradient controlled crystal growth from TIPS pentacene-poly(alpha-methyl styrene) blends for improving performance of organic thin film transistors. Org. Electron. 32, 195–199 (2016)
S. Bi, Z.R. He, J.H. Chen, D.W. Li, Solution-grown small-molecule organic semiconductor with enhanced crystal alignment and areal coverage for organic thin film transistors. AIP Adv. 5, 077170 (2015)
J.H. Chen, M. Shao, K. Xiao, Z.R. He, D.W. Li, B.S. Lokitz, D.K. Hensley, S.M. Kilbey, J.E. Anthony, J.K. Keum, A.J. Rondinone, W.Y. Lee, S.Y. Hong, Z.A. Bao, Conjugated polymer-mediated polymorphism of a high performance, small-molecule organic semiconductor with tuned intermolecular interactions, enhanced long-range order, and charge transport. Chem. Mater. 25, 4378–4386 (2013)
T. Ohe, M. Kuribayashi, R. Yasuda, A. Tsuboi, K. Nomoto, K. Satori, M. Itabashi, J. Kasahara, Solution-processed organic thin-film transistors with vertical nanophase separation. Appl. Phys. Lett. 93, 3 (2008)
J. Kang, N. Shin, D.Y. Jang, V.M. Prabhu, D.Y. Yoon, Structure and properties of small molecule-polymer blend semiconductors for organic thin film transistors. J. Am. Chem. Soc. 130, 12273–12275 (2008)
Z.R. He, D.W. Li, D.K. Hensley, A.J. Rondinone, J.H. Chen, Switching phase separation mode by varying the hydrophobicity of polymer additives in solution-processed semiconducting small-molecule/polymer blends. Appl. Phys. Lett. 103, 113301 (2013)
M.B. Madec, D. Crouch, G.R. Llorente, T.J. Whittle, M. Geoghegan, S.G. Yeates, Organic field effect transistors from ambient solution processed low molar mass semiconductor-insulator blends. J. Mater. Chem. 18, 3230–3236 (2008)
D.T. James, B.K.C. Kjellander, W.T.T. Smaal, G.H. Gelinck, C. Combe, I. McCulloch, R. Wilson, J.H. Burroughes, D.D.C. Bradley, J.S. Kim, Thin-film morphology of inkjet-printed single-droplet organic transistors using polarized Raman spectroscopy: effect of blending TIPS-pentacene with insulating polymer. ACS Nano 5, 9824–9835 (2011)
X.R. Li, W.T.T. Smaal, C. Kjellander, B. van der Putten, K. Gualandris, E.C.P. Smits, J. Anthony, D.J. Broer, P.W.M. Blom, J. Genoe, G. Gelinck, Charge transport in high-performance ink-jet printed single-droplet organic transistors based on a silylethynyl substituted pentacene/insulating polymer blend. Org. Electron. 12, 1319–1327 (2011)
S.Y. Cho, J.M. Ko, J. Lim, J.Y. Lee, C. Lee, Inkjet-printed organic thin film transistors based on TIPS pentacene with insulating polymers. J. Mater. Chem. C 1, 914–923 (2013)
P.F. Moonen, B. Vratzov, W.T.T. Smaal, G.H. Gelinck, M. Peter, E.R. Meinders, J. Huskens, A common gate thin film transistor on poly(ethylene naphthalate) foil using step-and-flash imprint lithography. Org. Electron. 12, 2207–2214 (2011)
M.B. Madec, P.J. Smith, A. Malandraki, N. Wang, J.G. Korvink, S.G. Yeates, Enhanced reproducibility of inkjet printed organic thin film transistors based on solution processable polymer-small molecule blends. J. Mater. Chem. 20, 9155–9160 (2010)
Y.B. Yuan, G. Giri, A.L. Ayzner, A.P. Zoombelt, S.C.B. Mannsfeld, J.H. Chen, D. Nordlund, M.F. Toney, J.S. Huang, Z.N. Bao, Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 5, 4005 (2014)
M. Park, Y. Min, Y.J. Lee, U. Jeong, Growth of long triisopropylsilylethynyl pentacene (TIPS-PEN) nanofibrils in a polymer thin film during spin-coating. Macromol. Rapid Commun. 35, 655–660 (2014)
Z.R. He, J.H. Chen, J.K. Keum, G. Szulczewski, D.W. Li, Improving performance of TIPS pentacene-based organic thin film transistors with small-molecule additives. Org. Electron. 15, 150–155 (2014)
J.H. Chen, S. Subramanian, S.R. Parkin, M. Siegler, K. Gallup, C. Haughn, D.C. Martin, J.E. Anthony, The influence of side chains on the structures and properties of functionalized pentacenes. J. Mater. Chem. 18, 1961–1969 (2008)
J.E. Anthony, J.S. Brooks, D.L. Eaton, S.R. Parkin, Functionalized pentacene: improved electronic properties from control of solid-state order. J. Am. Chem. Soc. 123, 9482–9483 (2001)
Z. He, Z. Zhang, S. Bi, Nanoscale alignment of semiconductor crystals for high-fidelity organic electronics applications. Appl. Nanosci. (2019). https://doi.org/10.1007/s13204-019-01068-4
Z.R. He, J.H. Chen, Z.Z. Sun, G. Szulczewski, D.W. Li, Air-flow navigated crystal growth for TIPS pentacene-based organic thin-film transistors. Org. Electron. 13, 1819–1826 (2012)
J.H. Chen, C.K. Tee, M. Shtein, J. Anthony, D.C. Martin, Grain-boundary-limited charge transport in solution-processed 6,13 bis(tri-isopropylsilylethynyl) pentacene thin film transistors. J. Appl. Phys. 103, 114513 (2008)
J.H. Chen, C.K. Tee, M. Shtein, D.C. Martin, J. Anthony, Controlled solution deposition and systematic study of charge-transport anisotropy in single crystal and single-crystal textured TIPS pentacene thin films. Org. Electron. 10, 696–703 (2009)
Y.H. Kim, J.E. Anthony, S.K. Park, Polymer blended small molecule organic field effect transistors with improved device-to-device uniformity and operational stability. Org. Electron. 13, 1152–1157 (2012)
Z.R. He, S. Shaik, S. Bi, J.H. Chen, D.W. Li, Air-stable solution-processed n-channel organic thin film transistors with polymer-enhanced morphology. Appl. Phys. Lett. 106, 183301 (2015)
Z.A. Lamport, K.J. Barth, H. Lee, E. Gann, S. Engmann, H. Chen, M. Guthold, I. McCulloch, J.E. Anthony, L.J. Richter, D.M. DeLongchamp, O.D. Jurchescu, A simple and robust approach to reducing contact resistance in organic transistors. Nat. Commun. (2018). https://doi.org/10.1038/s41467-018-07388-3
C. Liu, Y. Xu, Y.Y. Noh, Contact engineering in organic field-effect transistors. Mater. Today 18, 79–96 (2015)
R.M. Lu, Y. Han, W.M. Zhang, X.X. Zhu, Z.P. Fei, T. Hodsden, T.D. Anthopoulos, M. Heeney, Alkylated indacenodithieno 3,2-b thiophene-based all donor ladder-type conjugated polymers for organic thin film transistors. J. Mater. Chem. C 6, 2004–2009 (2018)
D.H. Kim, D.Y. Lee, H.S. Lee, W.H. Lee, Y.H. Kim, J.I. Han, K. Cho, High-mobility organic transistors based on single-crystalline microribbons of triisopropylsilylethynyl pentacene via solution-phase self-assembly. Adv. Mater. 19, 678–682 (2007)
G. Murtaza, I. Ahmad, H.Z. Chen, J.K. Wu, Study of 6,13-bis(tri-isopropylsilylethynyl) pentacene (TIPS-pentacene crystal) based organic field effect transistors (OFETs). Synth. Met. 194, 146–152 (2014)
D.H. Kim, D.Y. Lee, S.G. Lee, K. Cho, High-mobility organic single-crystal microtubes of soluble pentacene semiconductors with hollow tetragonal structures. Chem. Mater. 24, 2752–2756 (2012)
Z. He, Z. Zhang, S. Bi, Long-range crystal alignment with polymer additive for organic thin film transistors. J. Polym. Res. (2019). https://doi.org/10.1007/s10965-10019-11842-10961
Acknowledgements
S. Bi would like to thank Dalian University of Technology, China DUT16RC(3)051.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Z., Zhang, Z., Asare-Yeboah, K. et al. Poly(α-methylstyrene) polymer and small-molecule semiconductor blend with reduced crystal misorientation for organic thin film transistors. J Mater Sci: Mater Electron 30, 14335–14343 (2019). https://doi.org/10.1007/s10854-019-01803-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01803-1