Abstract
In this work, [Eu(tta)3(4-picNO)2] and [Eu(dbm)3(4-picNO)] complexes were incorporated on different concentrations (x = 1, 3, 5, 10 and 15%) in PMMA polymeric matrix (4-picNO: 4-Methylpyridine N-oxide) by the solvent casting method, yielding transparent and highly luminescent polymeric films. These materials were analyzed by X-ray diffraction, scanning electron microscopy and by energy dispersive, ultraviolet–visible spectroscopy, luminescence and vacuum ultraviolet–ultraviolet spectroscopies. The luminescence spectra of doped PMMA films are in agreement with an efficient intramolecular diketonate (tta) ligand-to-europium energy transfer. Furthermore, the values of experimental intensity parameters (Ω2,4) for luminescent polymeric materials were quite similar to those ones for isolated complexes, indicating that there is a homogeneous dispersion of Eu3+ complexes in the polymeric matrix, preserving their chemical and structural features. These behavior were also observed from the CIE diagram that show great similarity between the (x,y) coordinates for the doped PMMA samples compared to the isolated β-diketonate complexes with a reddish color tuning. The photostability investigation of the doped PMMA polymeric films and Eu3+ complexes has been also reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Interest in a variety of luminescent materials containing trivalent rare earth (RE3+) ions as emitting centers has grown significantly in recent years due to their electronic structures. However, their unique spectroscopic and magnetic properties emerge with distinguished importance when developing new materials for a wide variety of application fields [1,2,3]. The spectroscopic properties of RE3+ ions are inclined towards developments to luminescent markers [4], LEDs and OLEDs lighting devices [5], upconversion [6], solar cells sensitizers [7,8,9], as well as temperature sensing [10] and luminescent probes to biomolecules [11].
The 4f electrons interact weakly with the environment, resulting in little variance on the energy level structure on RE3+ ions. Therefore, the absorption and emission spectra of rare earth compounds exhibit narrow bands derived from 4f-4f transitions in solution or solid state, which facilitates the interpretation of these spectroscopic data [12]. The molar absorption coefficients of the 4f-intraconfigurational transitions are very low because they are forbidden by Laporte’s rule [13]. However, several RE3+ complexes often showcase a very strong luminescence arising from the metal ion. This phenomenon is explained by intramolecular energy transfer (ET) process from the organic ligand excited states to the RE3+ energy levels [13].
Besides, the Eu3+ ions are often used as a spectroscopic probe regarding the structural and photoluminescence properties [14]. As the first excited state (5D0) and the fundamental state (7F0) are non-degenerate, it greatly facilitates the analysis of experimental luminescence spectra. Moreover, 5D0 → 7FJ transitions with low J values and well separated 5D0 → 7FJ lines further aid spectral interpretation.
In this scenario, β-diketonate complexes have been largely employed due to their high molar absorptions and the position of the ligand triplet states (T1), which are slightly above the emitting excited levels of RE3+ ions, leading to an efficient luminescence sensitization. In addition, the photoluminescence processes can be supported by ancillary ligands with absence of high energy oscillators (i.e. vibrionic O–H oscillators present in hydrated complexes) that minimize the non-radiative contributions [12, 14].
Several RE3+ compounds present low thermal stability, limited photostability and poor mechanical properties. However, a potential approach to incorporate those complexes into organic polymers may solve most of these deficiencies, providing additional advantages such as flexibility, optical quality and moderate processing conditions [2, 15,16,17].
Poly(meyhylmethacrylate) (PMMA) polymer is widely known for its high light transmittance, chemical resistance, refractive index tailorability, low optical absorption and UV resistance. PMMA embedding organic and inorganic particles have been used to improvement of electrical conductivity, photoconductivity, photoluminescence, photo-induced charge-transfer and magnetic properties. [18,19,20,21,22,23,24].
In this work, [Eu(dbm)3·4-picNO] and [Eu(tta)3(4-picNO)2] complexes were synthesized by the precipitation method and further incorporated into PMMA polymeric film in different concentrations (x = 1, 3, 5, 10 and 15%) via solvent casting. The doped PMMA materials show high luminescence intensities, under UV irradiation. The experimental intensity parameters (Ωλ) for the complexes and doped systems are discussed. These novel luminescent Eu3+ materials may act as light converting molecular devices. The photostability of the doped PMMA polymeric films are higher than for the individual Eu3+ complexes. The generation of new light emitters with range color coordinates (x, y) for application as optical markers is presented.
2 Experimental section
2.1 Synthesis
All materials used in this work were purchased from Synth, VETEC, CAAL and Sigma-Aldrich and used without further purification. The purity of reagents follows: Ethyl alcohol (99.5%); Chloroform, anhydrous, contains amylenes as stabilizer (99%); Acetone (99.5%); Ammonium hydroxide solution 28.0–30.0% NH3 basis; 1,3-Diphenyl-1,3-propanedione or dibenzoylmethane (98%); 2-Thenoyltrifluoroacetone (99%); 4-Methylpyridine N-oxide (4-picNO) (98%); Europium(III) chloride hexahydrate (99,9%); Poly(methyl methacrylate) powder, analytical standard, average molecular weight of 78,000 g/mol by GPC (gel permeation chromatography).
2.1.1 Synthesis of Eu(β-diketonate)3·y(H2O) complexes
The hydrated europium β-diketonate complexes were carried out by the dissolution of dibenzoylmethane (dbm) or thenoyltrifluoroacetone (tta) in ethanol at 50 °C, followed by the dropwise addition of NH4OH until neutral pH was reached. The solutions were kept under constant stirring as the following addition of a stochiometric amount of an EuCl3·6H2O aqueous solution lowered the pH of the mixture. NH4OH was further added to neutralize pH once again. The mixtures were kept under stirring for 2 h until the precipitation of a characteristic yellowish solid. These compounds were then filtered and dried under reduced pressure in vacuum desiccator.
2.1.2 Synthesis of Eu(β-diketonate)3·y(4-picNO) complexes
This step aims to substitute water molecules in the Eu3+ coordination polyhedron of the previously synthesized hydrated complexes by 4-picoline-N-oxide (4-picNO) ligand. Thus, acetone solutions of Eu3+(dbm or tta) hydrated complexes were kept under stirring while a stoichiometric volume of 4-picNO acetone solution with defined concentration was added dropwise to the mixture. The precipitate powder was obtained by solvent evaporation and further dried under reduced pressure in vacuum desiccator.
2.1.3 Preparation of PMMA:[Eu(tta)3(4-picNO)2] (x%) films
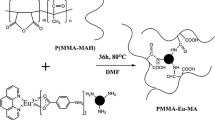
The preparation of the luminescent doped polymeric films was carried out by the solvent casting method. Firstly, a set amount of PMMA was kept under stirring in chloroform at 60 °C for 1 h. After the polymer dissolved, an acetone solution containing the stoichiometric amount of the previously synthesized [Eu(diketonate)3·y(4-picNO)] (diketonate: tta or dbm) was added in the mixture, which was stirred for an additional hour at 60 °C. The mixture was then transferred to a Petri dish, where the polymer film was obtained after evaporation of excess solvent at 40 °C (Fig. 1). The same procedure was used for several doping different concentrations of each Eu3+ β-diketonate complexes where x = 1, 3, 5, 10 and 15% weight for PMMA:[Eu(tta)3(4-picNO)2](x%) labeled as PET1, PET3, PET5, PET10 and PET15 and for PMMA:[Eu(dbm)3(4-picNO)](x%) labeled as PED1, PED3, PED5, PED10 and PED15, respectively.
2.2 Instrumental
Scanning electron microscopy (SEM) measurements were performed on HITACHI TM3000 microscope at 5 and 15 kV acceleration voltages in charge-up reduction mode, while Energy Dispersive Spectroscopy (EDS) data was obtained through a XFlash 430H detector and processed by Quantax70 software on the same equipment. X-Ray Diffraction (XRD) patterns were obtained by KαCu radiation on a Rigaku Miniflex II diffractometer. Measurements were made on the 5–80°(2θ) range, using a 0.03° step width under 1 s count time, as well as a 30 kV voltage and a 15 mA current. Ultraviolet–visible absorption spectroscopy (UV–vis) data were acquired by a Shimadzu UV-2600 spectrophotometer in the spectral range of 200 to 600 nm. Standard luminescence analysis was performed on a HORIBA Fluorog-3 Spectrofluorimeter and processed by FluorEssence software. Spectral acquisition was measured by an axial setup at an angle of 22.5° with slit widths of 0.5–1.0 nm and a blazed holographic grating 1200 lines/mm. Emission spectra was registered through a CCD detector while excitation spectra were measured by a photomultiplier with 1 nm step width. Synchrotron radiation measurements were obtained at the Brazilian Synchrotron Light Laboratory (LNLS), on TGM (Toroidal Grating Monochromator) beamline, which is dedicated to ultraviolet spectroscopy techniques in the energy range of 3 to 330 eV. Vacuum Ultraviolet to Ultraviolet (VUV-UV) spectroscopy was performed at room temperature within the 4.5–7.5 eV range, using a 200 nm thick quartz slide to avoid second order harmonics from the beamline. The resulting signal was collected by an optical fiber and further corrected applying sodium salicylate as a standard reference.
3 Results and discussion
3.1 Characterization
SEM images of the (a) [Eu(tta)3(4-picNO)2] and the [Eu(dbm)3(4-picNO)] complexes, PED3 and PET3 samples show distinguishable dispersion throughout the polymer from the microstructures, where it is noticeable the existence of complex aggregates in higher magnification images (Fig. 2). Moreover, it is plausible to assume a similarity in dimension sizes between the particle distribution on polymer samples and on isolated Eu3+β-diketonate complexes. Additionally, it is possible to observe the typical porous morphology regarding amorphous PMMA in high magnification of polymer samples.
EDS density maps in Fig. 3 indicate a successful dispersion of the β-diketonate complexes throughout the polymer, containing carbon, oxygen and europium. The europium imaging (Fig. 3d) suggest that Eu complexes are incorporate homogeneously in PMMA polymer matrix.
X-ray diffraction patterns display broadened diffraction peaks, revealing samples of an amorphous nature as expected for PMMA polymeric systems (Fig. 4). Furthermore, there are no significant changes in the diffraction pattern profiles of the polymer when increasing the doped concentration of both [Eu(tta)3(4-picNO)2] and the [Eu(dbm)3(4-picNO)] complexes from 1, 3, 5, 10 and 15% is seemingly identical on all analyzed samples, regardless of the doping β-diketonate complex.
3.2 Spectroscopic properties
UV–Vis absorption spectra of the PMMA, PET1 and PED1 samples present a clear distinction in the among the broad absorption bands (at around 225, 270 and 345 nm) originated from the β-diketonate ligands in the Eu3+ complexes and PMMA polymer (Fig. 5). The absorption spectral profiles of doped samples strongly differ from isolated PMMA, showing that the PET1 and PED1 samples present higher absorption intensity than for PMMA polymeric matrix. Moreover, it is shown that the coordination of the 4-picNO ligand does not result in any spectral changes compared to know europium β-diketonate coordination compounds, as its absorption range lies within the range of β-diketonate ligands, such as indicated by the absorption spectra.
VUV-UV excitation spectra by synchrotron radiation of the PMMA and doped films (PET10 and PED10) were recorded in the energy range from 4.5 to 7.5 eV (276–165 nm). The results show a qualitative understanding of ligand absorption processes at high energy (Fig. 6). As it can be seen, the excitation spectra of doped polymer samples presented a much higher absorption rate compared with PMMA matrix.
In addition, VUV-UV emission spectra present the same 5D0 → 7FJ transitions (J = 1, 2, 3, 4) under excitation at higher energies without any noticeable emission spectral changes (Fig. 6a–b). However, higher emission intensity of the 4f-4f transitions in the visible region are directly correlated to higher absorption energy illustrated in 3D spectra. This optical feature is observed for the PED10 doped film that presented maxima absorption bands around 4.5, 4.7, 5.2 and 6.4 eV, as well as for PET10 doped film shows higher absorption bands at 4.5, 4.7 and 6.0 eV.
Excitation spectra of PMMA:[Eu(tta)3(4-picNO)2](x%) and PMMA:[Eu(dbm)3(4-picNO)](x%) films (x = 1, 3, 5, 10 and 15%), monitoring on Eu3+ hypersensitive transition (~ 612 nm) were recorded at room temperature (300 K), in Fig. 7. The broad absorption bands are attributed to the intraligand transitions (S0 → Sn) [13] in the range of 200 to 425 nm. In addition, it is observed the narrow absorption with the lowest intensity assigned to 7F0 → 5D2 transition [14]. It is noticeable that the excitation spectra presents higher absorption intensity bands centered on the ligand compared to 4f-4f transitions, indicating its potential to act as an antenna on energy-transfer processes to Eu3+ ions. The comparison between the excitation spectra of 4-picNO and the hydrated europium of β-diketonate complexes reveals that the ancillary ligand coordination leads to the different excitation spectral profiles from the broad absorption bands assigned the S0 → Sn transitions (Figure S1).
Emission spectra of PMMA:[Eu(tta)3(4-picNO)2](x%) and PMMA:[Eu(dbm)3(4-picNO)](x%) films were recorded at 300 K, under excitation at 7F0 → 5L6 transition (394 nm) of Eu3+ ion, which show the narrow emission bands attributed to 5D0 → 7FJ (J = 0, 1, 2, 3 and 4) transition (Fig. 8) [14]. Among these optical data, the 5D0 → 7F0 transition is of extreme importance since it can indicate the number of symmetry sites around europium ions. However, the 5D0 → 7F1 transition is allowed my magnetic dipole, thus formally insensible to the chemical environment around the Eu3+ ion. In addition, the hypersensitive 5D0 → 7F2 and 5D0 → 7F4 transitions is deeply affected by the surrounding ligand field, as their emission intensity varies highly according to Eu3+ symmetry site.
Thus, a presence of the highest emission intensity bands assigned to 5D0 → 7F2 transition indicate that Eu3+ ions occupy non-centrosymmetric sites for all samples (Fig. 8). Moreover, the emission bands from 5D0 → 7F0 transition show only one emission peak, suggesting that the Eu3+ ion occupies just one single site of symmetry on PMMA samples. It is also noted that broad emission bands were not observed on spectral range from 450 to 600 nm (Fig. 8), indicating an efficient energy transfer from β-diketonates and 4-picNO organic ligands to the Eu3+ ion. Therefore, the Eu3+ coordination of the 4-picNO ligand is relevant regarding luminescence intensity, acting as an efficient antenna for energy transfer processes and prevents non-radiative decay channel due to OH oscillators from the hydrated complexes. In addition, the 4-picNO ligand exhibits different emission spectral profiles manly from the 5D0 → 7F2 transitions, indicating different chemical environment around europium ion due to different ligand field interaction in each coordination compound. Also, the coordination of the ancillary ligand enhances the energy transfer processes of Eu3+ complexes (Figure S1).
The experimental intensity parameters (Ωλ) consider the simultaneous contributions from forced electric dipole and dynamic coupling mechanisms and may be obtained from 4f-4f transitions in the absorption or emission spectra [12, 13]. The experimental intensity parameters Ω2 and Ω4 were determined for the [Eu(tta)3(4-picNO)2] and [Eu(dbm)3(4-picNO)] complexes, as well as for doped PMMA samples, in which, emission intensity is given in terms of the area under the curve of 5D0 → 7FJ transitions, being defined as:
where \(\hbar \omega_{{0 \to {\text{J}}}}\) corresponds to the transition’s energy, \({\text{N}}_{0}\) is the population of the emitting level (5D0), and \({\text{A}}_{{0 \to {\text{J}}}}\) represents the coefficient of spontaneous emission (emission intensity measured as emitted power; however, actually most equipment measures the ratio between absorbed and emitted number of photons). For experimental determination of the rates \({\text{A}}_{{0 \to {\text{J}}}}\),. the 5D0 → 7F1 transition allowed by magnetic dipole was used as standard reference, since it is formally insensitive to the chemical environment [12]. Thus, \({\text{A}}_{{0 \to {\text{J}}}}\) values are obtained from:
where \({\text{S}}_{{0 \to {\text{J}}}}\) is the area (integrated intensity) under the curve of the corresponding 5D0 → 7FJ transition of the 5D0 → 7F1 and 5D0 → 7F2,4 transitions. Hence, Ωλ (λ = 2, 4) presented on Table 1 were calculated from the following equation:
where \(\chi = {\text{n}}_{0} \left( {{\text{n}}_{0}^{2} + 2} \right)^{2} /9\) is the Lorentz local field correction, and \({\text{n}}_{0}\) is as the material’s refractive index. The squared reduced matrix elements are tabulated in the literature as 0.0032 and 0.0023 for J = 2 and 4, respectively.
The Ω2 and Ω4 experimental intensity parameter values of the [Eu(tta)3(4-picNO)2] complex compared with those doped polymer system (PET1, PET3, PET5, PET10 and PET15) as well as for [Eu(dbm)3(4-picNO)] compound compared with those PED1, PED3, PED5, PED10 and PED15 systems are quite similar (Table 1). These spectroscopic results suggest that the Eu3+ complexes maintain their chemical and structural features in the first coordination sphere when doped in PMMA polymeric film. indicating that the europium complexes do not interact straightly with the polymer in the first coordination sphere, but they are only dispersed into the polymeric matrix, though, of course, structural distortions may occur. Besides, emission spectral profiles of a particular Eu3+ (β-diketonate) complex (tta or dbm) are very similar compared with the respective doped polymer systems (Fig. 8), corroborating that the Eu3+ ion also act as powerful luminescence probe.
It is noted that know Ω2 values of Eu3+β-diketonate hydrated complexes [12] are within range of the Ω2 values obtained after the coordination of the 4-picNO ligand. Since the substitution of water molecules of hydrated complexes by the 4-picNO ligand occur by the coordination of O2−Eu3+, there is little structural effect around the metal ions, resulting in similar Ω2 values.
The photostability investigation of the luminescent materials [Eu(tta)3(4-picNO)2], [Eu(dbm)3(4-picNO)] complexes as well as PMMA:[Eu(tta)3(4-picNO)2](10%) and PMMA:[Eu(dbm)3(4-picNO)](10%) doped polymer films were evaluated (Fig. 9). Their emission spectra were recorded every 30 min for 2.5 h, under continuous exposure to UV irradiation at 394 nm, in the spectral range from 604 to 632 nm assigned to the 5D0 → 7F2 transition. 450 W Xenon lamp was used as excitation source, which is much more intense than the UV radiation from the Sun.
Figure 9 illustrates that the photostability of the Eu3+ β-diketonate complex containing dbm and 4-picNO ancillary ligand is higher compared to the tta ligand. In addition, the PED10 and PET10 doped polymeric films are more stable under UV irradiation than the individual europium complexes, indicating that PMMA:[Eu(dbm)3(4-picNO)](10%) doped film exhibit the highest photostability between the prepared luminescent materials [17, 25].
The higher photostability of PED10 and PET10 doped films than the individual complexes is probably due to the modification of the polymer surface and elimination of the defect within the texture/matrix after the UV light exposure, whereas for the Eu3+ ion, in the tta complex, the photodecomposition is the dominant process.
The question (mechanisms) of degradation, under UV (A, B or C) excitation of Ln3+ β-diketonates ligands, with ancillary ligands, is still an open question [25]. However, at least in the present case, we observe that the novel Eu3+ β-diketonate complexes with ancillary ligands as 4-picNO doped in PMMA films show considerably higher photostability then the complexes even under high power lamp UV-irradiation (450 W).
The CIE chromaticity diagram (Commission internationale de l’éclairage) illustrates the gradual spectral shifting of the Eu3+ complexes and doped PMMA polymeric materials [26], being directly related to emission spectral profiles and relative intensities of each samples (Fig. 10). By the CIE plot, it is possible to perceive a small shifting in (x,y) coordinates towards the reddish spectral region with increasing doped concentration of [Eu(tta)3(4-picNO)2] in polymeric films. This reddish shift is attributed to structural distortion around the metal ion, and possibly the nephelauxetic effect. Covalency effects, besides structural ones as mentioned, are certainly responsible for this observation. This is well discussed in references [27, 28]. Additionally, due to color tunneling, the PMMA doped films coordinates on the CIE slightly differ from the corresponding complexes due to small structural distortions around the metal ion.
CIE chromaticity diagrams showing the emission color tuning of PMMA:[Eu(tta)3(4-picNO)2](x %) and PMMA:[Eu(dbm)3(4-picNO)](x %) doped film (x = 1, 3, 5, 10 and 15%). Photographs of the luminescent materials complexes taken with a digital camera displaying the reddish emissions under UV irradiation at 366 nm
Finally, it is possible to identify different (2 J + 1) components of the 5D0 → 7F2 transition with increasing concentration on the luminescence spectra of PMMA doped samples. Although these components do not alter Ω2 values on a significant manner, their effects can be seen by the (x,y) coordinates shift presented in the CIE diagram.
4 Conclusion
Luminescent materials were successfully prepared by incorporation of the Eu3+β-diketonate complexes in PMMA films by the solvent casting method, showing amorphous nature. SEM/EDS analysis revealed the effective dispersion of Eu3+β-diketonate complexes throughout the polymer and the porous nature of PMMA. Furthermore, VUV-UV synchrotron radiation excitation, absorption and excitation measurements indicated the distinction imposed by the PMMA and β-diketonate ligands (tta and dbm). Luminescence spectra of all samples exhibit only emission peaks assigned to the 5D0 → 7FJ transitions of the Eu3+ ion with absence of phosphorescence of organic ligands, indicating efficient energy-transfer processes ligand–metal. The similar Ωλ parameter values meaning that the Eu3+ complexes maintain their chemical and structural features in the first coordination sphere when doped in PMMA film, suggesting that there is a dispersion between Eu3+ complexes and polymeric matrix. The doped PMMA polymeric films exhibit higher photostability then the complexes even under high power lamp UV-irradiation. The CIE diagram also revealed a great similarity between the (x,y) coordinates for the PMMA doped samples compared to the corresponding β-diketonate complex, exhibiting the reddish color tuning.
References
J.-C.G. Bunzli, On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 293–294, 19–47 (2015)
L.D. Carlos, R.A.S. Ferreira, V. de Zea Bermudez, S.J.L. Ribeiro, Lanthanide-containing light-emitting organic–inorganic hybrids: a bet on the future. Adv. Mater. 21(5), 509–534 (2009)
V.S. Sastri, J.C. Bunzli, J.R. Perumareddi, V.R. Rao, G.V.S. Rayudu, Modern aspects of rare earths and their complexes (Elsevier, Amsterdam, 2003)
B.K. Gupta, D. Haranath, S. Saini, V.N. Singh, V. Shanker, Synthesis and characterization of ultra-fine Y2O3:Eu3+ nanophosphors for luminescent security ink applications. Nanotechnology 21(5), 055607 (2010)
J. Kido, H. Hayase, K. Hongawa, K. Nagai, K. Okuyama, Bright red light-emitting organic electroluminescent devices having an europium complex as an emitter. Appl. Phys. Lett. 65(17), 2124–2126 (1994)
S. Heer, K. Kompe, H.U. Gudel, M. Haase, Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 16(23–24), 2102–2105 (2004)
D.-C. Yu, R. Martín-Rodríguez, Q.Y. Zhang, A. Meijerink, F.T. Rabouw, Multi-photon quantum cutting in Gd2O2:Tm3+ to enhance the photo-response of solar cells. Light 4(10), e3441–e3448 (2015)
J.-C. Bunzil, S.V. Eliseeva, Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 28(6), 824–842 (2010)
A. Nadort, J. Zhaob, E.M. Goldys, Lanthanide upconversion luminescence at the nanoscale: fundamentals and optical properties. Nanoscale 8(27), 13099–13130 (2016)
O.A. Savchuk, J.J. Carvajal, C.D.S. Brites, L.D. Carlos, M. Aguilo, F. Diaz, Upconversion thermometry: a new tool to measure the thermal resistance of nanoparticles. Nanoscale 10(14), 6602–6610 (2018)
J.-C.G. Bunzil, Lanthanide light for biology and medical diagnosis. J. Lumin. 170, 866–878 (2016)
G.F. Sá, O.L. Malta, C.D. Donega, A.M. Simas, R.L. Longo, P.A. Santa-Cruz, E.F. Silva Jr., Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord. Chem. Rev. 196(1), 165–195 (2000)
H.F. Brito, O.L. Malta, M.C.F.C. Felinto, E.E.S. Teotonio, In patai series, in The chemistry of metal enolates, ed. by J. Zabicky (John Wiley & Sons Ltd, New Jersey, 2009), pp. 131–184
K. Binnemans, Interpretation of europium (III) spectra. Coord. Chem. Rev. 295, 1–45 (2015)
P. Leanaerts, K. Driensen, R.V. Deun, K. Binnemans, Covalent coupling of luminescent Tris(2-thenoyltrifluoroacetonato)lanthanide(III) complexes on a merrifield resin. Chem. Mater. 17(8), 2148–2154 (2005)
S. Biju, M.L.P. Reddy, A.H. Cowley, K.V. Vasudevan, 3-Phenyl-4-acyl-5-isoxazolonate complex of Tb3+ doped into poly-bhydroxybutyrate matrix as a promising light-conversion molecular device. J. Mater. Chem. 19(29), 5179 (2009)
J. Kai, M.C.F.C. Felinto, L.A.O. Nunes, O.L. Malta, H.F. Brito, Intermolecular energy transfer and photostability of luminescence-tuneable multicolour PMMA films doped with lanthanide-β-diketonate complexes. J. Mater. Chem. 21(11), 3796–3802 (2011)
Q.D. Ling, D.J. Liaw, C. Zhu, D.S.H. Chan, E.T. Kang, K.G. Neoh, Polymer electronic memories: materials, devices and mechanisms. Prog. Polym. Sci. 33(10), 917–978 (2008)
S. Jana, A.S. Khojin, W.H. Zhong, H. Chen, X. Liu, Q. Huo, Effects of gold nanoparticles and lithium hexafluorophosphate on the electrical conductivity of PMMA. Solid State Ionics 178(19–20), 1180–1186 (2007)
H. Althues, R. Palkovits, A. Rumplecker, P. Simon, W. Sigle, M. Bredol, U. Kynast, S. Kaskel, Synthesis and characterization of transparent luminescent ZnS:Mn/PMMA nanocomposites. Chem. Mater. 18(4), 1068–1072 (2006)
S. Li, M.S. Toprak, Y.S. Jo, J. Dobson, D.K. Kim, Bulk synthesis of transparent and homogeneous polymeric hybrid materials with ZnO quantum dots and PMMA. Adv. Mater. 19(24), 4347–4352 (2007)
S.C. Farmer, T.E. Pattern, Photoluminescent polymer/quantum dot composite nanoparticles. Chem. Mater. 13(11), 3920–3926 (2001)
J. Gao, C. Lü, X. Lü, Y. Du, APhen-functionalized nanoparticles-polymer fluorescent nanocomposites via ligand exchange and in situ bulk polymerization. J. Mater. Chem. 17(43), 4591–4597 (2007)
R. Chai, H. Lian, P. Yang, Y. Fan, Z. Hou, X. Kang, J. Lin, In situ preparation and luminescent properties of LaPO4:Ce3+, Tb3+ nanoparticles and transparent LaPO4:Ce3+, Tb3+/PMMA nanocomposite. J. Colloid Interface Sci. 336(1), 46–50 (2009)
T.A. Kovacs, M.C.F.C. Felinto, T.B. Paolini, B. Ali, L.K.O. Nakamura, E.E.S. Teotonio, H.F. Brito, O.L. Malta, Synthesis and photoluminescence properties of [Eu(dbm)3·PX] and [Eu(acac)3·PX] complexes. J. Lumin. 193, 98–105 (2018)
C.S. Cunha, M. Köppen, H. Terraschke, G. Friedrichs, O.L. Malta, N. Stock, H.F. Brito, Luminescence tuning and single-phase white light emitters based on rare earth ions doped into a bismuth coordination network. J. Mater. Chem. C 6, 2668–12678 (2018)
O.L. Malta, H.J. Batista, L.D. Carlos, Overlap polarizability of a chemical bond: a scale of covalency and application to lanthanide compounds. Chem. Phys. 282, 21–30 (2002)
R.T. Moura Jr., O.L. Malta, R.L. Longo, The chemical bond overlap plasmon as a tool for quantifying covalency in solid state materials and its applications to spectroscopy. Int. J. Quantum Chem. 111, 1626–1638 (2011)
Acknowledgements
The authors thank the Brazilian Agencies: FAPESP, CAPES, CNPq, CNEN for financial support. Dr Veronica C. Teixeira, Dr Douglas Galante and Mr Leonardo M. Kofukuda (TGM beamline) from the Brazilian Synchrotron Light Laboratory, Brazilian Center for Research in Energy and Materials (LNLS and CNPEM), Campinas, SP, Brazil, are gratefully acknowledged for their assistance during vacuum UV-excited luminescence experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Francisco, L.H.C., Felinto, M.C.F.C., Brito, H.F. et al. Development of highly luminescent PMMA films doped with Eu3+β-diketonate coordinated on ancillary ligand. J Mater Sci: Mater Electron 30, 16922–16931 (2019). https://doi.org/10.1007/s10854-019-01639-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01639-9