Abstract
In this study, pristine and Zr-doped CeO2 nanoparticles with chemical formula Ce1−xZrxO2 (x = 0, 0.05, 0.075 and 0.1) have been prepared through facile hydrothermal process. The influence of Zr doping on the microstructure, thermal, optical and photocatalytic properties of CeO2 was systematically explored through various analytical techniques. Analysis of the XRD data reveals cubic fluorite structure of the samples with average crystallite size of 12, 15, 21 and 30 nm respectively for different Zr doping. The optical properties of the nanoparticles were studied through UV–visible absorption and photoluminescence (PL) spectroscopy. X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy measurements were performed to examine the chemical state and microstructure of the synthesized materials. The functional groups and mode of vibrations have been identified by the Fourier transform infrared (FTIR) spectroscopy. A reduction in the optical band gap of CeO2 (from 3.25 to 3.10 eV) is observed on systematic Zr doping. In addition, significant enhancement in the photocatalytic performance is also noticed for the doped samples (92.2%) as compared to the pristine one (68.7%) for the degradation of methylene blue (MB) dye under visible light irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, nanostructure semiconductors have attracted much interest owing to their novel features different from their bulk counterpart [1]. These innovative characteristics of nanomaterials may find promising applications in photoelectronics, catalysis, optics, magnetic devices etc. [2,3,4,5,6,7,8]. Among the various semiconductors, ceria oxide (CeO2) is one of the important rare earth oxides due to its exceptional technological applications in solar cells [9], solid oxide fuel cell electrolyte materials [10], oxygen storage capacity [11], catalysts [12], oxygen storage materials, optoelectronic and electrochemical devices [13]. Moreover, it is known that CeO2 is very reactive, strong oxidizing agent and has wide band gap with different morphologies, that are utilized as photocatalyst in environmental purification, three-way catalysts for the elimination of toxic auto-exhaust gases, oxygen permeation membrane systems, UV blockers, biological applications [14,15,16,17,18,19,20]. However, it may worth to mention few disadvantage of catalysts in operating for different processes under different temperature as compared to the other catalysts. In fact, firstly, the temperature inside the catalytic reactor needs to be initially low in order to allow the adsorption of the pollutants onto the porous substrate at high temperatures, and then the pollutants will be released from the porous substrate. Secondly, the temperature needs to increase to allow the oxidation of the pollutants and to regenerate the adsorptive property of the porous substrate. The limit of adsorption and the competitive effect of the involved pollutants need to be carefully investigated in order to avoid their release into the environment. It is reported that the band gap and other essential properties of nanoscale CeO2 can be considerably modified by the doping of suitable dopants associated with the formation of defect states between valence band and conduction band, i.e. red shift of UV spectra [21]. In order to improve its catalytic performance, zirconium has been widely used to form Ce–Zr solid solution [22]. The oxidative performance of ceria-based catalysts is closely related to the oxygen vacancies and species. Hori et al. [23] have reported three to five times higher oxygen storage capacity due to zirconium doping in CeO2. However improved thermal resistance and redox properties of Zr doped ceria were observed by Adamowska et al. [24]. But, there is always a chance to improve the properties of existing material by various means and hence doping is one of the important routes in order to achieve new and better properties of nanostructure materials. In this context, substitution of Zr to form Ce1−xZrxO2 matrix alters the structural and electronic properties of CeO2. This enhances the chemical and thermal stability of the system than the pristine ceria [25,26,27]. A significant modification is also observed in the optical properties of doped CeO2, attributed to the transition of electrons between O 2p and the lower unoccupied energy level of the dopants. Moreover, enhancement in the degradation of organic pollutants will surely influence our environment that will be beneficial for human health. Several physical and chemical methods have been developed for the synthesis of CeO2 doped with different concentrations of Zr4+ ions, such as sol–gel [28], co-precipitation [29], hydrothermal processes [30], modified co-precipitation, flame pyrolysis [31] etc. Among all the methods, we have tried hydrothermal approach to synthesize pristine and Zr-doped CeO2 nanostructures. The prepared samples were characterized by various analytical techniques to investigate the influence of Zr doping on the physico-chemical properties of CeO2.
2 Experimental details
Nanoparticles of Ce1−xZrxO2 (x = 0, 0.05, 0.075 and 0.1) were synthesized via a simple hydrothermal process by varying the amounts of reactants. AR grade cerium and zirconium nitrates were used without purification. Typically, 5 mmol of cerium nitrate and 0.25 (5%), 0.375 (7.5%) and 0.50 (10%) mmol of zirconium nitrate were dissolved in 20 ml distilled water and kept on a magnetic stirrer until the salts completely dissolved. The pH value was adjusted to 7 by the addition of 0.1 M solution of NaOH during this reaction. Thereafter, product was placed in an autoclave with 100 ml polytetrafluoroethylene liner at 100 °C for 24 h. The obtained white precipitate was carefully washed with distilled water and ethanol to eliminate organic impurities. Then the precipitate was dried at 80 °C overnight followed by the calcination at 500 °C for 5 h in a muffle furnace to get nanoparticles of CeO2 and Zr doped CeO2 as final product.
The prepared samples were characterized using different analytical techniques. An XRD system (PANanalytical, X’Pert High) having CuKα radiation (λ = 1.5406 Å) was used to determine the crystal structure and phase purity of the samples in the 2θ range of 10°–100° at a scanning speed of 2o/min. The X-ray photoelectron spectroscopy (XPS) measurements were performed with monochromatic Mg Kα line (1253.6 eV) radiation source on an ESCA model VG 3000 system. Field emission scanning electron microscope (FE-SEM) was used to observe surface morphology of the samples. The Fourier transform infrared (FTIR) spectra were registered on PerkinElmer FTIR spectrometer in the wavenumbers 400–4000 cm−1. The micro-Raman and PL spectra were recorded at room temperature using Horiba Raman/PL system (IY-Horiba-T64000) having a continuous wave laser operating at a 325 nm. The optical properties were studied through UV–visible spectrophotometer (Shimadzu-2550, Japan) in the wavelength range of 300–900 nm. The photocatalytic degradation of Ce1−xZrxO2 (x = 0, 0.05, 0.075 and 0.1) nanoparticles was examined by the degradation of methylene blue (MB) organic dye in an aqueous solution under visible light irradiation produced by the 400 W Xenon lamp (λ ~ 400 nm). In a typical experiment, 50 mg undoped and doped samples were added to 100 ml solution (10 mg/L) of the MB dye and then stirred on a magnetic stir in the dark environment for 1 h to found adsorption/desorption equilibrium between the reactants and catalysts. The light source was placed above the samples in order to irradiate them in the normal direction. The distance between the samples and the light source was kept about 20 cm. During the process of the reaction, about 2 ml suspensions were taken and centrifuged and then filtered to remove the residual catalyst particulates for analysis. Thereafter, the change in MB concentration was monitored using Shimadzu UV-1800 spectrometer at a wavelength of 664 nm.
The degradation efficiency (η) of MB dye was evaluated by the following equation:
where C0 gives the initial concentration of the solution and C is the concentration after light irradiation.
3 Results and discussion
3.1 XRD
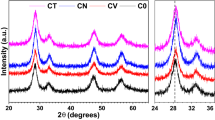
Phase purity and crystal structure of the prepared samples were examined through XRD patterns recorded at room temperature. These patterns for undoped and Zr doped CeO2 samples are shown in Fig. 1. All diffractograms contain ten main Bragg’s peaks that correspond to the (111), (200), (220), (311), (222), (400), (331), (420), (422) and (333) planes, located at angles (2θ) ~ 28.93, 33.44, 47.85, 56.74, 59.33, 69.67, 77.11, 79.37, 88.77 and 95.87° respectively. These peaks are well matched with the cubic fluorite-type phase of pure CeO2 having Fm3m space group (JCPDS no 34-0394) and unit cell parameter, a = 5.41 Å. No any additional peak corresponding to Zr related impurity phases was detected that confirms high purity and single phase of the samples.
The lattice parameters (a = b = c) of the undoped and Zr doped CeO2 nanostructures are estimated from the XRD patterns using the following equation [32]:
where dhkl is the interplanar spacing. The calculated values of the lattice parameters are given in Table 1. It is clear that the lattice parameter slightly varies by the doping of Zr ions in pristine CeO2 [32]. For the undoped sample, its value is very close to the theoretical result (a = 5.41 Å) that indicates possible replacement of Ce by the Zr ions in the cubic fluorite structure. Additionally, it is evident from the XRD patterns that the most intense diffraction peak (111) in Zr doped CeO2 slightly shift towards the higher angles (inset Fig. 1) that signifies that the Zr substitution affects/distort the crystal structure of the samples. This may be due to the difference in the ionic radii of zirconium and cerium ions.
The average crystallite size (t) is evaluated using the full width at half maximum (FWHM) of the most intense (111) peak by the Scherrer’s equation given as [33]:
where k is a constant equal to 0.94, β and θ are the FWHM and position of the most intense peak respectively. The obtained values of the crystallite size for undoped and Zr doped CeO2 NPs are given in Table 1.
3.2 XPS analysis
The surface composition and chemical states of Ce1−xZrxO2 samples were investigated through XPS measurements. Figure 2a shows a typical XPS survey spectrum of Ce0.95Zr0.05O2 sample. The core levels of Ce 3d, Ce 4p, Zr 3d, and O 1s have been classified in the spectrum. In the spectrum of Ce 3d, six peaks are observed and shown in Fig. 2b. Out of these, peaks appeared at 881.29, 888.10, and 897.9 eV assigned to the Ce 3d5/2 components, whereas remaining signals of Ce 3d3/2 could be seen at 906.1, and 915.8 eV. Two main peaks of Zr 3d are noticed at 181.6 and 183.9 eV associated with Zr 3d5/2 and Zr 3d3/2 respectively (Fig. 2c). This indicates 4+ oxidation state of Zr in Ce1−xZrxO2 matrix and in good agreement with the earlier reported work [34,35,36]. The O 1s XPS spectra show significant changes on Zr incorporation which is very clear in Fig. 2d and further revealed a notable shift in the XPS peak towards lower binding energy as compared to the pristine CeO2. The main peak is appeared at 531.26 eV attributed to the presence of oxygen atoms [37,38,39], while another peak obtained at 528.10 eV may be due to the chemisorption of oxygen caused by surface hydroxyl associated with the O–H bonds [40, 41].
3.3 SEM/HRTEM
In order to obtain more detailed information about the internal structure of the synthesized samples, electron microscopy measurements were carried out using FESEM/HRTEM and images are displayed in Fig. 3. The FESEM micrographs of pristine and 10% Zr doped CeO2 samples shown in Fig. 3a, b exhibit spherical shape and agglomeration of the nanoparticles in both the samples. It is also evident that the particles size increases in the doped sample which is consistent with the XRD results. Figure 3c, d shows HRTEM micrographs of the corresponding samples that signify good crystallinity and clear lattice fringes. The lattice fringe with interplanar spacing (d) of 0.31 nm is indexed to the XRD plane (111) of face-centered cubic structure of CeO2, whereas other fringes could be ascribed to the (111) crystal plane of the Zr doped nanostructures. The EDS spectra displayed in Fig. 4a, b are used to determine the elemental compositions of the pristine and Zr doped CeO2 NPs. It can be seen that only cerium and oxygen as the main species in pure CeO2, while well-defined peaks corresponding to the zirconium, cerium and oxygen elements are present in the Zr doped CeO2 NPs that established appropriate existence of the elements in the samples. The elements carbon and copper were detected from the TEM grid used.
3.4 FTIR
The vibrational characteristics of the prepared nanoparticles were studied through FTIR spectroscopy analysis in the wavenumbers 400 to 4000 cm−1 and shown in Fig. 5. The IR bands in the range of 400–650 cm−1 are the characteristic peak of ceria appeared in all the samples [42]. The other peaks between 1050 and 1630 cm−1 correspond to the Ce–O stretching vibrations attributed to the adsorbed water on ceria surface [43]. Because of the large surface-to-volume ratio of the nanomaterials, the water molecules are normally absorbed onto the surface from the surrounding environment [41]. The band at 2350 cm−1 indicates the C–H stretching mode of the methylene groups. However, the absorption peak cantered at 3422 cm−1 is associated with the O–H stretching vibrations of water molecules [43].
3.5 Raman spectroscopy
It is an excellent technique to get microstructural information of materials. Room temperature Raman spectra of undoped and Zr doped CeO2 samples in the wavenumbers 200 to 2500 cm−1 are depicted in Fig. 6. A sharp peak at 465 cm−1 appeared in all samples could be associated with the F2g mode of fluorite type cubic structure analogous to the symmetric stretching mode of the oxygen atoms around Ce4+ ions [44]. The other peaks at 1237, 1351, 1397, 1481 and 1644 cm−1 are attributed to the oxygen vacancies generated by the compensation of charge carriers due to the induced defects by the doping ions in CeO2 lattice [45]. The outcomes of Raman analysis are consistent with the XRD results.
3.6 Optical studies
The influence of doping ion on the optical properties was investigated through UV–vis absorption spectroscopy. Figure 7a shows the UV–vis absorption spectra of pristine and Zr doped CeO2 nanoparticles in the wavelengths between 300 and 900 nm. The absorption spectrum of pristine sample shows a broad peak at a wavelength of 327 nm that signifies slightly blue shift as compared to its bulk counterpart (388 nm) [46]. It indicates the onset of the absorption edge in nanostructures. Further, a red shift in the absorption is observed for the Zr-doped CeO2 samples. This may be credited to the change in surface to volume ratio of the nanoparticles related to the alteration in the crystallite size or morphology of the nanoparticles. The bandgap (Eg) of the samples is evaluated by employing the Tauc’s relation given as [47]:
here α is the absorption coefficient related to the absorbance (A) and thickness of the cuvette (x) as α = 2.303A/x, and B refers a constant that is independent on photon energy. The value of m depends on the type of optical transition of the semiconductor i.e. m = 2 for direct and 1/2 for an indirect transition [48]. The intercept of the linear part of the plot between (αhv)2 and hν is used to calculate the value of Eg for undoped/doped CeO2 nanoparticles and illustrated in Fig. 7b. The value of the bandgap is given in Table 1 for all the samples. It indicates that the bandgap of synthesized ceria nanoparticles (Eg ~ 3.25 eV) is slightly higher than its bulk form (Eg ~3.20 eV). As particles size decreases, the density of states becomes more quantized and bandgap of the CeO2 shift towards the higher value. Thus, a blue shift in the absorption band is observed. On the other hand, subsequent doping of Zr ions in CeO2 reduces the band gap of the system. This may be attributed to the formation of localized states within the bandgap associated with the oxygen vacancies or enhancement in the density of Ce ions [49, 50]. Hence a red shift occurs either ions substitution or interfacial polaron mechanism due to the electron–phonon coupling. This has resulted reduction in the bandgap of the system and theoretically interpreted by the second-order perturbation theory [51].
3.7 Photoluminescence spectroscopy
Photoluminescence (PL) is a non-destructive technique generally used for the study of photogenerated electron–hole pairs in semiconductors [52]. Figure 8 shows the room-temperature PL spectra of pristine and Zr-doped CeO2 nanoparticles excited by a source of wavelength 330 nm. The spectra present emission peaks in the spectral range of 350–650 nm for the pristine and doped samples that exhibit almost identical emission bands, suggested the relative oxygen vacancies in the samples. The emission peaks between 350 and 600 nm are ascribed to the charge transfer transition from Ce 4f to O 2p levels [53]. The doping ion introduces energy levels just below the 4f band of ceria associated with the defects due to the oxygen vacancies. These levels are affected by the temperature and density of defects in material, and hence electron excitation mostly occurs from defects level to O 2p level at room temperature [41, 54]. The earlier reports have similar results for CeO2 nanostructures [55]. In the present study, a considerable improvement in the PL emission is noticed for the undoped CeO2 in comparison of Zr doped samples that indicates fast recombination rate of electrons and holes in the undoped sample. Therefore, these results confirmed that the doping of Zr ions could delay the recombination rate of photogenerated charge carriers.
3.8 Photocatalytic activities
The photocatalytic performance of the synthesized photocatalysts (pristine and Zr doped CeO2 NPs) were assessed through the deterioration of MB dye under visible light irradiation and shown in Fig. 9. It is noticed that the intensity of characteristic absorption peak of MB dye centred at ~ 664 nm constantly reduces with increase in irradiation time and almost disappeared after 150 min due to degradation of the dye molecules. In order to explore the photocatalytic activities of the samples, the dye concentration as a function of reaction time is studied and displayed in Fig. 9a–e. The variation in concentration of MB dye with irradiation time is shown in Fig. 10 for all studied samples. It is noted that the degradation efficiency of the dye for pure and doped samples is found to be 68.7, 75.8, 80.3 and 92.2% respectively after 150 min irradiation. All Zr doped samples exhibit higher activity as compared to the undoped CeO2 NPs under visible light irradiation. The enhancement of photocatalytic activity of Zr-doped CeO2 is attributed to the red shift in the optical adsorption edge on Zr doping in CeO2 matrix. When Zr is doped in ceria, it behaves as a sensitizer to absorb visible light. The excited electrons of CeO2 move to the conduction band of Zr and equal numbers of holes generated in the valence band. In addition, photo-induced electrons transferred from the conduction band to the energy level of dopant ion i.e. Zr4+ for CeO2–Zr system. While the holes generated in the valence band move to the energy level of –O–Zr–O– for CeO2–Zr. This behaviour of charge carriers resulted the higher value of photocatalytic performance for the Zr doped samples than undoped CeO2.
The mechanism for the enhancement in photocatalytic activity of Zr-doped CeO2 is depicted schematically in Fig. 11 and can be explained as: the slow recombination of electron–hole pairs (as confirmed from PL measurements) resulted in the charge separation at the interface and reach to the photocatalyst surface by diffusion. Generally, recombination of electron–hole pair mainly depends on the PL emission intensity and the lower intensity confirms a decrease in the recombination rate that is beneficial for enhancing photocatalytic activity of catalyst. Another reason of enhancement in the photocatalytic activity can be attributed to the effect of Zr-doping on the optical properties of CeO2 which help to narrowing the band gap of the matrix and promoting to the improvement of visible light photocatalytic activity of Zr-doped CeO2. The possible degradation mechanism of the MB dye through Zr doped CeO2 already available in literature, however brief description is shown in Fig. 11. When the prepared semiconductor is irradiated by visible light, an electron from valance band (VB) excited to the conduction band (CB) leaving a hole in valance band. Generally, recombination of these electron–holes reduces the photocatalytic activity of the CeO2. But Zr present in the material, trap the electron from CB of CeO2 that inhibits the electron–hole recombination. It is well established that ‘Zr’ traps the electrons from CB of CeO2. Therefore, Zr doping suppresses the electron–hole recombination by the electron trapping [42]. The electrons trapped by Zr produce more number of superoxide radical anions and at the same time VB holes of CeO2 react with water to produce highly reactive hydroxyl (OH) radical. The superoxide radical anion and hydroxyl radical facilitate the degradation of MB dye.
4 Conclusions
The pristine and Zr doped CeO2 nanoparticles for different concentration of dopant were prepared by a facile simple hydrothermal process. Electron microscopy images revealed uniform and spherical shape of the nanoparticles. HRTEM exhibits crystalline nature of the sample. XPS study confirms that the Zr and Ce ions exist in 4+ state in the doped CeO2. Analysis of the optical properties measurements established the presence of oxygen vacancies/defects in the Zr doped CeO2 samples that make the material suitable for visible light absorption associated with the reduction in band gap. It was observed that the Zr doped nanoparticles have enhanced photocatalytic response as compared to pure CeO2 for the degradation of MB dye under visible light irradiation. The results predict that the synthesized samples may have promising application water treatment.
References
J. Dai, M.L. Bruening, Nano Lett. 2, 497–501 (2002)
C.T. Campbell, C.H. Peden, Science 309, 713–714 (2005)
A. Abbasi, D. Ghanbari, M. Salavati-Niasari, M. Hamadanian, J. Mater. Sci.: Mater. Electron. 27, 4800–4809 (2016)
M.G. Sujana, K.K. Chattopadyay, S. Anand, Appl. Surf. Sci. 254, 7405–7409 (2008)
H. Safajou, H. Khojasteh, M. Salavati-Niasari, S. Mortazavi-Derazkola. J. Colloid Interface Sci. 498, 423–432 (2017)
L. Wang, F. Meng, Mater. Res. Bull. 48, 3492–3498 (2013)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, M. Hamadanian. RSC Adv. 5, 33792 (2015)
M. Salavati-Niasari, F. Soofivand, A. Sobhani-Nasab, M. Shakouri-Arani, A. Yeganeh Faal, S. Bagheri, Adv. Powder Technol. 27, 2066–2075 (2016)
H. Yu, Y. Bai, X. Zong, F.Q. Tang, G.Q. Lu, L.H. Wang, Chem. Commun. 48, 7386–7388 (2012)
P. Singh, N.Q. Minh, Int. J. Appl. Ceram. Technol. 1, 5–15 (2004)
G. Cheng, J. Xiong, F.J. Stadler, Powder Technol. 249, 89 (2013)
J. Zhou, L. Zhao, Q. Huang, R. Zhou, X. Li, Cat. Lett. 127, 277–284 (2009)
J. Zhang, H. Kumagai, K. Yamamura, S. Ohara, S. Takami, et al. Nano Lett. 11, 361–364 (2011)
R. Bakkiyaraj, G. Bharath, K. Hasini Ramsait, A. Abdel-Wahab, E.H. Alsharaeh, S.M. Chen, M. Balakrishnan, RSC Adv. 6, 51238–51245 (2016)
V. Matolin, M. Cabala, I. Matolinova, M. Skoda, M. Vaclavu, K.C. Prince, T. Skala, T. Mori, H. Yoshikawa, Y. Yamashita, S. Ueda, K. Kobayashi, Fuel Cells 10, 139–144 (2010)
P. Jasinski, T. Suzuki, U.H. Anderson, Sens. Actuators B 95(1–3), 73–77 (2003)
K.S. Brinkman, H. Takamura, H.L. Tuller, T. Iijima, J. Electrochem. Soc. 157, B1852–B1857 (2010)
D. Wang, Y. Kang, V. Doan-Nguyen, J. Chen, R. Kungas, N.L. Wieder, K. Bakhmutsky, R.J. Gort, C.B. Murray, Angew. Chem. 50, 4378–4381 (2011)
A. Corma, P. Atienzar, H. Garcia, J.Y. Chane-Ching, Nat. Mater. 3, 394–407 (2004)
K.S. Hemalatha, K. Rukmani, RSC Adv. 6, 74354–74366 (2016)
K.S. Ranjith, P. Saravanan, S. Chen, C. Dong, C.L. Chen, S. Chen, K. Asokan, R. Thangavelu, R. Kumar, J. Phys. Chem. C 118, 27039 (2014)
Y. Liu, C. Wen, Y. Guo, G. Lu, Y. Wang, J. Phys. Chem. C 114, 9889–9897 (2010)
C.E. Hori, H. Permana, K.Y. Simon Ng, A. Brenner, K. More, K.M. Rahmoeller, D. Belton, Appl. Catal. B Environ. 16, 105–117 (1998)
M. Adamowska, S. Muller, P. Dacosta, A. Krzton, P. Burg, Appl. Catal. B Environ. 74, 278–289 (2007)
G. Zhou, P.R. Shah, T. Kim, P. Fornasiero, R. Gorte, J. Catal. Today 123, 86–93 (2007)
P. Fornasiero, R. Dimonte, G.R. Rao, J. Kaspar, S. Meriani, A. Trovarelli, M. Graziani, J. Catal. 151, 168–177 (1995)
M. Boaro, C. de Leitenburg, G. Dolcetti, A. Trovarelli, J. Catal. 193, 338–347 (2000)
P. Periyat, F. Laffir, S.A.M. Tofail, E. Magner, RSC Adv. 1, 1794–1798 (2011)
P. Venkataswamy, K.N. Rao, D. Jampaiah, B.M. Reddy, Appl. Catal. B 162, 122–132 (2015)
Z. Wang et al., Appl. Catal. B 138–139, 253–259 (2013)
W.J. Stark, M. Maciejewski, L. Madler, S.E. Pratsinis, A. Baiker, J. Catal. 220, 35–43 (2003)
M.A. Wahab, S.S. Physics, Narosa Publishing House, New Delhi, 2nd edn, 2010
M.A. Majeed Khan, W. Khan, A. Kumar, A.N. Alhazaa, Mater. Lett. 219, 269–272 (2018)
V.B. Kanattukara, K. Dong-Kyu, P. Dae-Won, Nanoscale 2, 1222–1228 (2010)
S. Tsunekawa, K. Asami, S. Ito, M. Yashima, T. Sugimoto, Appl. Surf. Sci. 252, 1651–1656 (2005)
J. Xin Liu, X. Ding, R. Lin, Z. Gao, W.-L. Li, Dai, Appl. Catal. A 503, 117–123 (2015)
Y. Yu, L. Zhong, J. Ding, W. Cai, Q. Zhong, RSC Adv. 5, 23193–23201 (2015)
D. Jampaiah, S.J. Ippolito, Y.M. Sabri, B.M. Reddy, S.K. Bhargava, Catal. Sci. Technol. 5, 2913–2924 (2015)
D. Jampaiah, S.J. Ippolito, Y.M. Sabri, J. Tardio, P.R. Selvakannan, A. Nafady, B.M. Reddy, S.K. Bhargava, Catal. Sci. Technol. 6, 1792–1803 (2016)
Y.-W. Zhang, R. Si, C.-S. Liao, C.-H. Yan, J. Phys. Chem. B 107, 10159–10167 (2003)
M.A.M. Khan, W. Khan, M. Ahamed, A.N. Alhazaa, Sci. Rep. 7, 12560 (2017)
R. Bakkiyaraj, M. Balakrishnan, G. Bharath, N. Ponpandian, J. Alloy. Compd. 724, 555–564 (2017)
M. Ornatska, E. Sharpe, D. Andreescu, S. Andreescu, Anal. Chem. 83, 4273–4280 (2011)
J. Lin, L. Li, Y. Huang, W. Zhang, X. Wang, A. Wang, T. Zhang, J. Phys. Chem. C 115, 16509 (2011)
A. Martnez-Arias et al., J. Catal. 240, 1–7 (2006)
H. Zhang et al., Environ. Sci. Technol. 45, 3725–3730 (2011)
S. Kumar, M.A. Majeed Khan, J. Mater. Sci. Technol. 29(12), 1151–1155 (2013)
M.A. Majeed Khan, M.W. Khan, M. Alhoshan, M.S. AlSalhi, A.S. Aldwayyan, M. Zulfequar, Mater. Lett. 64, 1929–1932 (2010)
C. Hu, Z. Zhang, H. Liu, P. Gao, Z.L. Wang, Nanotechnology 17, 5983–5987 (2006)
M.E. Khan, M.M. Khan, M.H. Cho, Sci. Rep. 7, 5928 (2017)
I. Khan, S. Khan, R. Nongjai, H. Ahmed, W. Khan, Opt. Mater. 35, 1189–1193 (2013)
M.A. Majeed Khan, S. Kumar, T. Ahamad, A.N. Alhazaa, J. Alloy. Compd. 743, 485–493 (2018)
S. Kumar, A. Kumar, Mater. Sci. Eng. B 223, 98–108 (2017)
S.K. Alla, E.V.P. Komarala, R.K. Mandal, N.K. Prasad, Mate. Chem. Phys. 182, 280–286 (2016)
G. Wang, Q. Mu, T. Chen, Y. Wang, J. Alloys Compd. 493, 202–207 (2010)
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through Group Project No. RGP-1437-023.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majeed Khan, M.A., Khan, W., Naziruddin Khan, M. et al. Enhanced visible light-driven photocatalytic performance of Zr doped CeO2 nanoparticles. J Mater Sci: Mater Electron 30, 8291–8300 (2019). https://doi.org/10.1007/s10854-019-01147-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01147-w