Abstract
Electroless nickel–phosphorus films were produced on silicon substrates in alkaline bath solutions, with the addition of thiourea in a concentration range of 1.0–5.0 mg L−1. The influence of thiourea on the chemical composition, morphology and corrosion resistance of the films was studied. The results revealed thiourea had a major influence on plating rate, phosphorus-content and aggregate size. The optimal content of thiourea was 1 mg L−1. Thiourea accelerated the deposition rate at low concentration of 1 mg L−1, but deceased the deposition rate and the phosphorus content at high concentration. The surface of the film without thiourea was smooth and dense. Also, with increasing thiourea content, the surface evolved into coarse nodular morphology with clear intercolonial boundaries. With the addition of 1 mg L−1 thiourea, the film had better corrosion resistance compared to film without thiourea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electroless nickel (EN) has been used in many applications such as electronics, chemical processing, automotive and other industries. Electroless deposition of metals could produce nanostructures on semiconductor silicon wafers because of its simplicity and ability to fill in fine patterns. The mass production of metallic nanostructure on silicon is one of the key techniques in micro and nanoscale device applications [1]. The stability of EN bath is a very critical factor in industrial applications. In order to stabilize EN plating solutions, it is necessary to add additives in the baths. Organic additive is of importance in the electrochemical and chemical electroless deposition processes [2,3,4,5], which can affect the catalytic activity of the substrate surface due to the formation of complexes [6]. Stabilizers prevent decomposition of EN plating baths [7]. There are two types of stabilizers [8]. One is a substitution type; where metal ions such as Pb2+ are deposited on the active surface of substrates through displacement reaction and could inhibit the occurrence of the random reduction of Ni2+ ions. The other is an adsorption-poisoning type. Some anodic ion stabilizers, such as sulphide and iodide ions can inhibit EN deposition via adsorption on the catalytic sites of the substrate surface.
Thiourea and lead acetate are the commonly used stabilizers in EN baths [8]. However, the use of Pb2+ ions causes environmental pollution due to the toxicity. Therefore, thiourea has been proposed as a substituent for Pb2+ ions. Thiourea could influence EN plating process in many ways [9,10,11,12], such as co-deposition of sulphur, microstructure and performance of EN deposits. The effect of thiourea on electroless Ni–P layer on metallic substrates in an acid environment has been reported [8, 13,14,15,16,17,18]. However, a few works in the published literature report the effect of stablizers on EN layers on semiconductor silicon wafers in alkaline bath solutions. Baskaran et al. [8] studied the effects of thiourea, succinic acid and lead acetate on electroless Ni–P deposited on copper substrates; results showed that thiourea accelerated deposition rate and enhanced crystallinity of the film at low concentration, but inhibited deposition rate at high concentration. Sotskaya et al. [19, 20] reported the influence of organic additives containing sulphur ions on electroless Ni–P films, and concluded that the greater the electron-donor properties, the higher their adsorbability and the stronger the blocking effect, which could stabilize the electrolyte. Cheong et al. [21] investigated the effect of stabilizers on the bath stability of EN deposition; concluding that thiourea influenced chemical composition and morphology of the deposits. At high concentration > 2 mg L−1, the P-content was decreased to < 7 wt%. Liu et al. [22] found that thiourea had influence on morphology and corrosion resistance of EN layers, and the addition of thiourea resulted in a decrease in P-content of the deposits.
In our previous published literature [23, 24], the effects of bath chemistry and plating parameters on the EN layers deposited on semiconductor silicon wafers were studied in an alkaline bath solution. EN deposition onto semiconductor surface is complicated due to the band structure of the semiconductor, which affects both the thermodynamics and the kinetics of metal deposition processes [25]. In experimental procedure, the EN baths were highly sensitive to high temperature, high pH and the concentration of reducing agents. The precipitates may be formed in NE baths, and resulted in bath decomposition. Therefore, it is necessary to add stabilizers in the baths in order to inhibit the decomposition reaction. The concentration of the stabilizers is considered to be the most critical parameter since stabilizers can act as accelerators or inhibitors of the deposition depending on their concentration in the plating bath [8, 20, 21]. In this study, it is important to determine the effective concentration of stabilizers for alkaline bath solutions. We choose thiourea as a stabilizer in an alkaline solution and study the influence of thiourea on chemical composition, morphology and corrosion resistance of Ni–P films deposited on silicon wafers.
2 Experimental
The p-type monocrystalline silicon wafers were used as the substrates (size: 10 × 10 × 0.2 mm3). The substrates were etched by reactive ion etching process. Yoo et al. [26] has described this process to be effectively helpful towards improving utilization of absorbed light for silicon wafers. Prior to deposition, the substrates were immersed and etched for 30 s in a diluted HF solution (HF:H2O = 1:100), then rinsed and washed with deionized water, followed by cleaning with acetone in an ultrasonic bath for 5 min. An electrolytic cell was used for EN deposition. The bath composition and operating conditions for EN deposition are listed in Table 1. Thiourea (S=C(NH2)2) was used as the stabilizer to prevent decomposition of bath solutions. The pH value was adjusted to 9 ± 0.1 with diluted 5 mol L−1 sodium hydroxide solution. No agitation was employed to the bath during the plating process.
The weight gain of the specimens before and after each deposition was measured by an analytical balance (FA2004B, resolution 0.1 mg). The morphology of the top surface of the film was observed by a scanning electron microscopy (SEM, Gemini Sigma 300/VP) operated in a high-vacuum mode. The chemical composition of the film was determined by an attached liquid nitrogen-cooled Oxford Si X-ray energy dispersive spectroscopy (EDS) detector. In order to confirm uniformity, each sample was measured at three different locations and average values were measured.
Electrochemical properties of the substrate and the films were studied by electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization tests using an electrochemical workstation (CHI-660E, Shanghai, China). The as-coated specimen was connected to a copper wire by conductive adhesive film and then covered with epoxy resin leaving a square surface area of 1 cm2 exposed to the solution. The electrolyte was 3.5 wt% NaCl solution open to the air. The deionized water was from an ultra-pure water system equipment (Unique-R10, 18.25 MΩ cm resistivity at 25 °C). A standard three-compartment cell was used with an Ag/AgCl saturated KCl reference electrode, a Pt counter electrode and the as-coated specimen as working electrode. Prior to the beginning of EIS measurements, the working electrode was first immersed in the solution for 15 min in order to test the open circuit potential. Subsequently, EIS was measured and plotted in a frequency range between 100 kHz and 10 MHz. The amplitude of sinusoidal voltage was 5 mV. After EIS testing, the potentiodynamic current–potential curve was measured and recorded at a sweep rate of 20 mV min−1.
3 Results and discussion
3.1 Characterization
Figure 1 shows the plots of the P-content and the weight gain of the specimens as a function of thiourea contents. When the concentration of thiourea was 1 mg L−1, the weight gain reached the largest value. However, the P-content (10 wt%) almost remained unchanged compared with the film without thiourea. When the concentration of thiourea was more than 1 mg L−1, the weight gain obviously decreased, indicating a decrease in the deposition rate. The P-contents had similar trends. According to this data, the addition of thiourea resulted in an increase in the deposition rate at a content of 1 mg L−1 and decreased the plating rate and P-content at high concentration of > 1 mg L−1. Therefore, the best concentration of thiourea was 1 mg L−1. At high concentration of thiourea, Ni2+ ions on the surface of the catalytic active sites were occupied by many sulphur ions, reducing the possibility of Ni2+ ions adsorption on the surface of the substrate [14]. Ashtiani et al. [27] pointed that thiourea could prevent sodium citrate from controlling the rate at which free Ni2+ ions were released for reduction reaction. Thiourea might adsorb into the deposit. Too much adsorption of thiourea on the substrate or in the deposit certainly poisoned deposition reaction and decreased plating rate [10].
The P-content is one of the important factors, which could determine the structure of deposits. There were three different structures for electroless Ni–P films according to P-content in the deposits, namely: low (3–5 wt%), medium (6–9 wt%) and high (10–14 wt% and above) P deposits [11]. High P deposit is composed of an amorphous structure in nature and exhibits good corrosion resistance. In this study, the P-content in the film with thiourea at 1 mg L−1 was ~ 10 wt%, which was the same with the film without thiourea; indicating the film was high P deposit. However, as the content of thiourea was more than 1 mg L−1, the film was medium P deposit. Therefore, thiourea had a major effect on the deposition rate and the P-content in the film.
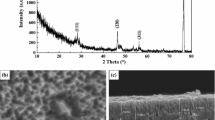
Figure 2 shows the SEM images of top surface of the films. No additive was used, the surface of the film was smooth and dense, but large aggregates were present on the surface (see Fig. 2a). With the addition of thiourea, the surface morphologies of the films were changed. As the content of thiourea was 1 mg L−1, the surface was dense, with no evidence of microcracks or pores, and the aggregates were round, regular and closely arranged on the surface (see Fig. 2b). The size of aggregates decreased with increasing thiourea. Figure 3 shows the influence of thiourea on particle size distribution for the surface of the film. From Fig. 3, a reduction in the number of large particles with increasing thiourea content can be observed. The percentage of small aggregates of size 0.25–5 µm increased significantly from 37 to 72%. With the increase of thiourea content, the surface was not smooth, compared with the film without thiourea (see Fig. 2a). At high concentration of thiourea, the coarse nodular morphology was evident (see Fig. 2c–e), with clear intercolonial boundaries. The deposition of Ni–P film with thiourea on silicon substrate follows a 3D island growth (Volmer–Weber) mechanism due to the low interaction energy between metal adatoms and the semiconductor surface [25]. Some islands began to coalesce each other during the deposition of the film (Fig. 2c–e). The relatively rough surface morphology was obtained in the presence of high thiourea concentration, compared with surface morphology of the film in the absence of thiourea. The surface morphology of the film with thiourea was in good agreement with the results reported in other literature [21, 22].
In alkaline solution, the chemical reactions for electroless Ni–P film without additives would be expressed by the following Eqs. (1)–(4) [24, 28]:
According to Eqs. (1)–(4), the hydrogen evolution reacted from the water and hydride ions. However, the surface of silicon could be oxidized in alkaline solution. The chemical reaction could be expressed as Eq. (5):
The galvanic displacement reaction occurred as the silicon wafers were immersed in alkaline solution [29]. It was a spontaneous reaction, which for Ni deposition could be expressed as Eq. (6):
During the spontaneous reaction, the surface of the substrate was oxidized and became a catalytic surface inducing further co-deposition of Ni–P film (see Fig. 4a). It was suggested that nickel was deposited by galvanic displacement with the silicon, and this reaction was accompanied by the formation of a silicon oxide [30, 31]. After the nucleation of nickel, the electrons released by the oxidation of silicon were supplied to Ni2+ ions through the deposited nickel metal, resulting in the growth of crystals of nickel metal. The oxide layer was produced as a result of the reaction of oxidized Si with OH− ions, which was promoted by Ni2+ ions. The surface of the substrate was transformed into a catalytic surface. When the catalytic surface formed, the oxidation and reduction reactions of \({{\text{H}}_2}{\text{PO}}_{2}^{ - }\) ions began to occur, followed by the co-deposition of Ni–P film. EN deposition on silicon substrates can be achieved by three processes [25]: electron transfer from the conduction band to metal ions in the solution, electron transfer from surface states to the solution and electron transfer from the valence band to the solution.
In this study, thiourea was added into the electrolyte. Some researchers [9, 19,20,21] suggested that thiourea exerted an accelerating or inhibiting effect on the deposition rate, mainly depending on the concentration of thiourea. The addition of thiourea could lower activation energy of chemical reaction for EN deposition [9]. When the content of thiourea was 1 mg L−1, an increase in plating rate was observed due to the enhancement in the reaction rate. When the concentration was more than 1 mg L−1, thiourea inhibited the deposition due to the surface coverage of the deposit by thiourea. The lowering activation energy of the deposition reaction was overwhelmed by the inhibiting effect of surface adsorption at high thiourea concentration. Xu et al. [12] proposed a two-step mechanism by which thiourea accelerated and inhibited EN deposition. Thiourea was oxidized into a reactive intermediate radical. In the first step, thiourea was oxidized into a thiourea free radical ([HN = C(NH2)S]*) through a charge transfer process. In the next step, the thiourea free radical reacted with another thiourea free radical to form the reactive intermediate, formamidine disulphide (HN = C(NH2)–S–S–(NH2)C = NH). The two-step reaction of thiourea facilitated the electrons transfer to Ni2+ ions and enabled acceleration of the plating rate, expressed by Eq. (7). The formamidine disulphide was subsequently reduced by hypophosphite and regenerates, this reaction process was represented by Eq. (8) [12].
According to Eqs. (7) and (8), thiourea reacted with Ni2+ and \({{\text{H}}_2}{\text{PO}}_{2}^{ - }\) ions. Figure 4b displays the schematic diagram of EN deposition with the addition of thiourea. When small amount of thiourea was added, the plating rate increased because of the catalysis process. As large amount of thiourea was added, more formamidine disulfide formed. When formamidine disulfide was generated in sufficient amounts, it is able to oxidize \({{\text{H}}_2}{\text{PO}}_{2}^{ - }\) to \({{\text{H}}_2}{\text{PO}}_{3}^{ - }\), which inhibited the reduction of Ni2+ by\({{\text{H}}_2}{\text{PO}}_{2}^{ - }\), and finally decreased the deposition rate. Baskaran et al. [8] suggested that the oxidation of \({{\text{H}}_2}{\text{PO}}_{2}^{ - }\) ion was dominant at low thiourea concentration while the reduction in the catalytic sites became dominant at high thiourea concentration. With continuous addition of thiourea, more thiourea adsorbed onto the substrate surface and further decreased the plating rate until all available thiourea binding sites were filled and the plating rate reached a minimum value.
3.2 Corrosion resistance
Figure 5 shows the typical impedance data of the substrate and electroless Ni–P films acquired by EIS shown in both the Nyquist and the Bode impedance plots. The effective concentration of thiourea for EN plating bath chemistry was 1 mg L−1, the corrosion resistance of the film with 1 mg L−1 thiourea was studied. All the Nyquist plots exhibited a capacitive behaviour at high frequencies. Whereas, the capacitive loops differed considerably in their size. The capacitive loops were related to the corrosion behaviour. The real impedance at which the imaginary part vanished for the capacitive part could provide an approximate comparison of the corrosion resistance of the substrate and Ni–P films. As shown in Fig. 5a, the substrate and the film without thiourea had smaller real impedance (inserted amplified plot) than the film with 1 mg L−1 thiourea. Thus indicating that the addition of 1 mg L−1 thiourea resulted in an increase in the corrosion resistance of Ni–P film. In Fig. 5b, the Bode impedance plot also confirmed the above results. The impedance modulus, |Z| at low frequencies, also called polarization resistance Rp, was often used to estimate the corrosion resistance [22, 32]. The Rp value at low frequencies of the film with thiourea was 22.8 KΩ cm2, whereas Rp values of the substrate and the film without thiourea decreased to 57.3 Ω cm2 and 1 KΩ cm2, respectively. The increase in Rp of the film with thiourea indicated that the addition of 1 mg L−1 thiourea increased the corrosion resistance of the film. It was noted that the P-content for the film in the presence of 1 mg L−1 thiourea was the same with that without thiourea, however the corrosion resistance of the former was better than the latter.
Figure 6 shows the polarization curves of the substrate and Ni–P films. The film with 1 mg L−1 thiourea had a significantly lower corrosion current density (Icorr) and more positive corrosion potential (Ecorr), compared with the substrate and the film without thiourea (see Table 2). The corrosion resistance of the film was related to Ecorr and Icorr. Thus, more positive Ecorr and lower Icorr for the film with 1 mg L−1 thiourea indicated better corrosion resistance. It’s known that the corrosion resistance of electroless Ni–P film is influenced strongly by the percentage of P-content in the deposit [11]. The high P deposits show better corrosion resistance than the low and medium P films. Although the P-content in the film with 1 mg L−1 thiourea was the same with that without thiourea, the addition of 1 mg L−1 thiourea improved the corrosion resistance of electroless Ni–P film due to high-P content in the film and co-deposition of elements like sulphur as well as the decrease in porosity [8].
4 Conclusions
Based on an electroless method, this study examines Ni–P films plated with the addition of thiourea on silicon substrates in alkaline bath solutions. The influence of thiourea on the chemical composition, morphology, deposition rate and corrosion resistance of the deposits were studied. The conclusions were made as follows:
-
1)
Thiourea had an influence on the plating rate and P-content in the Ni–P films. The optimal content of thiourea for the deposition of Ni–P film was 1 mg L−1. Thiourea accelerated the deposition rate at a low concentration of 1 mg L−1, but deceased the deposition rate of the film at the concentration more than 1 mg L−1. High content of thiourea was added, thiourea adsorbed on the surface of the substrate or the deposit, which could result in the reduction in the number of catalytic sites on the surface.
-
2)
The surface of the film in the absence of thiourea was smooth and dense. With increasing additive content, the size of the nodular was decreased, and the coarse nodular morphology was evident with clear intercolonial boundaries.
-
3)
With addition of 1 mg L−1 thiourea, the corrosion resistance of Ni–P film on silicon substrate could be improved due to the high-phosphorous content in the films and co-deposition of elements like sulphur as well as the decrease in porosity.
References
H. Seto, K. Hashizume, T. Murata, J. Japan Inst. Electron. Pack. 1, 21 (2018)
W.P. Wu, J.J. Jiang, P. Jiang et al., Appl. Surf Sci. 307, 434 (2018)
W.P. Wu, Z.Z. Wang, P. Jiang et al., J. Electrochem. Soc. D 164(14), 985 (2017)
W.P. Wu, Appl. Phys. A 1028–1036, 122 (2016)
W.P. Wu, Electrochemistry. 699, 84 (2016)
Y. Wang, H.P. Liu, S.F. Bi et al., RSC Adv. 9656, 6 (2016)
B.N. Hua, R.X. Sun, G. Yu et al., Surf. Coat. Technol. 84, 228 (2013)
I. Baskaran, T.S.N.S. Narayanan, A. Stephen, Mater. Chem. Phys. 117, 99 (2006)
K.P. Han, J.L. Fang, Int. J. Chem. Kinet. 259, 28 (1996)
K.L. Lin, J.W. Hang, Mater. Chem. Phys. 204, 76 (2002)
J. Sudagar, J. Lian, S. Wei, J. Alloys Compd. 183, 571 (2013)
H.W. Xu, J. Brito, O. Sadik, J. Electrochem. Soc. 816, 150 (2003)
M. Kunimoto, K. Endo, H. Nakai et al., Electrochim. Acta 311, 100 (2013)
R. Ambat, W. Zhou, Surf. Coat. Technol. 124, 179 (2004)
J. Li, P.A. Kohl, J. Electrochem. Soc. 631, 149 (2002)
U.S. Mohanty, B.C. Tripathy, S.C. Das et al., Metall. Mater. Trans. B. 737, 36 (2005)
K.P. Han, J.L. Fang, Metal Finish. 73, 95 (1997)
A. Hung, J. Electrochem. Soc. 1047, 132 (1985)
N.V. Sotskaya, E.I. Ryabinina, T.A. Kravchenko et al., Prot. Met. 250, 39 (2003)
N.V. Sotskaya, E.I. Ryabinina, T.A. Kravchenko et al., Prot. Met. 245, 39 (2003)
W.J. Cheong, B.L. Luana, D.W. Shoesmith, Appl. Surf. Sci. 282, 229 (2004)
H.P. Liu, N. Li, S.F. Bi et al., Thin Solid Films. 1883, 516 (2008)
N.M. Miao, J.J. Jiang, W.P. Wu, J. Nanomater. (2018) https://doi.org/10.1155/2018/1817542
W.P. Wu, J.J. Jiang, Appl. Nanosci. 325, 7 (2017)
G. Oskam, J.G. Long, A. Natarajan et al., J. Phys. D 1927, 31 (1998)
J. Yoo, G. Yu, J. Yi, Solar Energy Mater. Solar Cells. 2, 95 (2011)
A.A. Ashtiani, S. Faraji, S.A. Iranagh et al., Arab. J. Chem. 1541, 10 (2017)
Y.C. Zhang, N.H. Ru, X. Rong, Electroplating Handbook (National Defence Industry Press, Beijing, 2011)
H.F. Hsu, C.L. Tsai, C.W. Lee et al., Thin Solid Films 4786, 517 (2009)
N. Takano, N. Hosoda, T. Yamada, T. Osaka, J. Electrochem. Soc. 1407, 146 (1999)
D. Niwa, T. Homma, T. Osaka, J. Phys. Chem. B. 9900, 108 (2004)
A.R. Rahimi, H. Modarres, M. Abdouss, Surf. Eng. 367, 25 (2009)
Acknowledgements
This research was partly supported by the National Natural Science Foundation of China (Grant Nos. 61604021; 51875053), the Natural Science Foundation of Jiangsu Province (Grant No. BK20150260) and the Funding of Changzhou high technology research key laboratory of mould advanced manufacturing (Grant No. CM20173001).
Author information
Authors and Affiliations
Contributions
WPW designed the study and supervised an MSc student, JWL, who performed most of the experiments, but did not contribute to the preparation of this article. NYY, LXT, and QQW supervised the preparation and pretreatment process of silicon wafers and discussed it. NMM guided the corrosion experiment, JJJ did this experiment. LZ conducted the SEM/EDS experiments and assisted in their data analysis. WPW, YZ, and LZ discussed the results. WPW wrote the manuscript. WPW and YZ revised the manuscript. All authors approved the submission of the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, W., Liu, J., Miao, N. et al. Influence of thiourea on electroless Ni–P films deposited on silicon substrates. J Mater Sci: Mater Electron 30, 7717–7724 (2019). https://doi.org/10.1007/s10854-019-01088-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01088-4