Abstract
Cd1−xZnxS thin films have been cathodically electrodeposited on glass/fluorine-doped tin oxide substrates using a low-cost two-electrode configuration with an electrolytic bath containing cadmium chloride, zinc chloride and sodium thiosulphate precursors. The deposition was carried out by varying zinc chloride concentration (0 M, 0.3 M, 0.6 M and 0.9 M) in the deposition electrolyte. The structural, compositional, phonon vibrational, morphological and optical properties of the resulting films have been characterized using glancing incidence X-ray diffraction, energy-dispersive X-ray (EDX) spectroscopy, Raman spectroscopy, scanning electron microscopy (SEM) and UV–Vis spectrophotometry respectively. The XRD result indicates the existence of hexagonal structure of Cd1−xZnxS with intensities of the diffraction peaks decreasing as more Zn is incorporated into the film as a result of decrease in deposition rate. EDX results show the presence of Cd, Zn and S in the deposited Cd1−xZnxS thin films. SEM images show uniform and densely packed surface morphology of the films with distinct shaped grains which gradually less distinct as more Zn is incorporated into the films. The optical measurements reveal a significant increase in energy band gap of the Cd1−xZnxS material as more zinc is incorporated into the films and a film thickness decreases with the energy band gap increasing from 2.40 eV for CdS to 2.62 eV for Cd1−xZnxS. These results show that Cd1−xZnxS material can be applied as a better window material for CdTe, CIG(S,Se) and CZTS-based solar cells than CdS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
CdS and ZnS are renowned wide and direct band gap group II–VI semiconductors with bulk band gaps, (Eg) of 2.42 eV and 3.68 eV respectively [1, 2]. These band gap values are convenient when these materials are used as n-type window materials in CdTe, CuIn(S,Se) and Cu(In,Ga)Se photovoltaic solar cells [3,4,5,6,7]. Despite the fact that, CdS and ZnS are popularly used as window materials and as n-type heterojunction partners to p-type absorber materials, CdS is known to have high coefficient of absorption while ZnS is highly resistive [8, 9]. To minimize the solar absorption loss associated with the high absorption coefficient of CdS and the high resistivity associated with ZnS, ternary alloyed compounds like Cd1−xZnxS have attracted research interest due to the fact that the energy band gap of such materials can be tuned and the lattice parameters can be varied [10, 11]. Cd1−xZnxS thin film has properties in between CdS and ZnS, and it is formed by substituting Zn for Cd in CdS lattice. The addition of Zn improves optical properties of Cd1−xZnxS thin film for solar cell application in such a way that it reduces the optical absorbance and increase the transmittance as compared to CdS. This helps to reduce the window absorption loss associated with CdS. Other than for photovoltaic solar cells as a window material, Cd1−xZnxS compound has also been used for different applications such as in water treatment, optical filters, gas sensors, multilayer light emitting diodes (LEDs), field effect transistors, and other optoelectronic devices [12,13,14,15].
Various techniques such as electrodeposition [16], chemical bath deposition [17], chemical vapour deposition [18], vacuum evaporation [19], co-precipitation [20], spray pyrolysis [21], sol–gel [22], metal organic chemical vapour deposition (MOCVD) [23], pulsed laser deposition (PLD) [24], RF sputtering [25], and close space sublimation (CSS) [26] have been employed for the synthesis of Cd1−xZnxS thin films. Though, Cd1−xZnxS thin film can be deposited using these listed deposition techniques, using electrodeposition method has not been investigated adequately in order to explore the advantages of electrodeposition technique which include large area deposition, long bath lifetime, minimum waste generation, easy process control, and self-purification of electrolytic bath [27,28,29]. Hence in this paper electrodeposition technique has been used as a viable technique to obtain quality Cd1−xZnxS thin films. Typically, in the deposition of thin films using electrodeposition technique, the use of reference electrode is common. But nowadays, two electrode configuration has become promising by reducing the cost due to the reference electrode and avoiding possible contamination of the electrolytic solution in the case of reference electrode with leaky or broken frit [29, 30]. For these reasons, and the fact that small variations in deposition voltage does not have significant influence in the case of semiconductor deposition at least [29], a simple two-electrode deposition set-up is used in the present work. In electrodeposition technique, optimization of deposition conditions, such as electrolytic bath temperature, deposition time, pH, stirring, electrodes used, concentration of ions in the electrolytic bath and applied voltage, is essential to improve the physico-chemical properties of deposited films [27, 28]. Hence, by careful choice of these deposition conditions, in this paper, the influence of Zn concentration on the physico-chemical properties of Cd1−xZnxS thin films investigated using a simple two-electrode deposition configuration for possible solar cell application. This approach has not been reported yet.
2 Experimental details
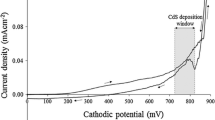
Cd1−xZnxS thin films were cathodically electrodeposited from aqueous electrolytic solution containing 0.3 M of cadmium chloride (CdCl2), as Cd source, different concentrations (0 M, 0.3 M, 0.6 M, and 0.9 M) of zinc chloride (ZnCl2), as zinc source and 0.03 M sodium thiosulphate (Na2S2O3) as sulphur source in 400 ml of deionised water. All the chemicals were laboratory reagent grade purchased from Sigma-Aldrich. Fluorine-doped thin oxide coated glass (glass/FTO) substrates were used for the deposition. All glass/FTO substrates were immersed in a diluted laboratory soap solution and cleaned ultrasonically for 30 min. Thereafter, these substrates were washed using acetone, ethanol and methanol while rinsing with de-ionized water in between washing, and finally dried in air. Figure 1 shows a schematic of the low-cost two-electrode deposition set-up, which was also used to carry out a cyclic voltammetry (CV) of the deposition electrolyte. For CV measurement and deposition of the films, a computerized Gill AC potentiostat (ACM instruments, United Kingdom) and high-purity graphite rod as counter electrode, have been used, with glass/FTO substrate as working electrode. Due to the fact that, S2O32− dissociates at relatively lower cathodic potential, sulphur comes to deposit first on the substrate from around cathodic potential of 400 mV and the current density is low. As seen in Fig. 2, as cathodic potential increases in the region between 1400 and 1600 mV, both sulphur and cadmium begin to co-deposit and a gradual increase in current density is observed. But, as the cathodic potential goes above 1600 up to 1900 mV, despite the fact that Zn2+ has relatively higher standard reduction potential of − 760 mV than Cd2+ (− 403 mV) and S2O32−, all sulphur, cadmium and zinc start to co-deposit and sharp increase in current density is observed in the cyclic voltammetry graph. The reaction mechanisms for the formation of CdS and CdZnS are presented as shown in Eqs. (1–3) starting with the dissociation of the thiosulphate ions in the presence of acid in the electrolyte. Hence, the possible cathodic deposition voltages for Cd1−xZnxS were found from the cyclic voltammogram to be between 1600 and 1900 mV. In-between these two voltages different preliminary depositions were done and − 1700 mV was settled for as the best deposition potential.
Then for formation of CdS on the cathode, we have
And for the formation of CdZnS, we have
pH of the electrolytic bath was settled at 3.5 ± 0.02, and dilute hydrochloric acid (HCl) and ammonium hydroxide (NH4OH) were used to control the pH. Moderate stirring rate of the electrolytic bath with a bath temperature of 85 °C and deposition time 30 min. were applied. Following this, the deposition of Cd1−xZnxS thin films was carried out using four pre-cleaned glass/FTO substrates at four different zinc chloride concentrations of 0 M, 0.3 M, 0.6 M and 0.9 M ZnCl2 with steps of 0.3 M in-between. Afterwards, all electrodeposited Cd1−xZnxS thin film samples were rinsed with de-ionized water and then dried in air. Finally, all electrodeposited Cd1−xZnxS thin films were annealed at 400 °C for 20 min for physical and chemical characterization. Hence, all the data reported in this paper are for the annealed samples since this is the best form of the materials for solar cell application.
For characterization, glancing incidence X-ray diffraction (GIXRD) was carried out using Rigaku Smartlab X-ray diffractometer (Rigaku Corporation, USA); scanning electron microscopy (SEM) was carried out using Tescan VEGA3 SEM machine (Tescan Corporation, Czech Republic); energy-dispersive X-ray (EDX) was carried out using the SEM machine with Oxford X-MaxN EDS detector attached to it; Raman spectroscopy was carried out using the alpha 300 R Confocal Raman machine (WITec, Germany) and UV–Vis spectrophotometry was carried out using a Shimadzu UV-1700PC spectrophotometer (Shimadzu, Corporation, Kyoto, Japan).
3 Results and discussion
3.1 Structural properties
X-ray diffraction measurements were carried out using glancing incidence X-ray diffraction (GIXRD) to study crystal structure and crystalline quality of the deposited Cd1−xZnxS thin films. It is known that, Cd1−xZnxS thin films can exist in cubic, hexagonal or mixed phase crystal structure based on preparation condition [1, 31, 32]. As shown in Fig. 3, the XRD patterns show the presence of only hexagonal phase peaks at all the different ZnCl2 concentrations used. These peaks occur at diffraction angles of 2θ = 24.92°, 26.56°, 28.22°, 43.84° and 48.04° corresponding to diffraction lattice planes of (100)H, (002)H, (101)H, (110)H and (103)H respectively as can be seen in the patterns of all the samples. These diffraction peaks of the samples compare very well with those of Joint Committee on Powder Diffraction and Standards (JCPDS) reference files 00-049-1302 for hexagonal CdZnS. Likewise, for pure CdS thin film (with 0 M ZnCl2) hexagonal CdS phase peaks are also observed at diffraction angles of 2θ = 24.89°, 26.62°, 28.24°, 43.84° and 47.70° corresponding to diffraction lattice planes of (100)H, (002)H, (101)H, (110)H and (103)H respectively which match JCPDS reference file 01-080-0006 for hexagonal CdS.
As can be seen from Fig. 3, at 0 M ZnCl2 concentration, the film (CdS) is very crystalline and even the FTO peaks are dwarfed by the high intensities of CdS peaks. But, as Zn is incorporated into CdS lattice, intensities of the major XRD peaks of Cd1−xZnxS [i.e. (100) and (101) peaks] start to decrease in relation to the FTO peaks. This decrease in intensity of Cd1−xZnxS thin film peaks, which also correspond with those of CdS, continues up to 0.6 M ZnCl2. At 0.9 M ZnCl2 the intensities of the XRD peaks are diminished considerably. It is known that, in electrodeposition technique ions are brought to the surface of the substrate by migration, convection and drift. Hence, when more ZnCl2 is added into the electrolytic solution the concentration of Zn ions in the bath will increase and this increases the possibility of more zinc getting incorporated into CdS lattice to form Cd1−xZnxS thin films. In addition, the presence of more Zn2+ in the electrolyte results in low deposition rate of CdZnS as will be seen later from the calculated deposition rates in Table 2. Therefore, a decrease in intensity of the peaks may happen largely due to reduced thickness of the resulting CdZnS films as a result of low deposition rate.
The crystallite sizes (D), from Scherrer formula, the inter-planar spacings (dhkl), from Bragg’s diffraction equation and lattice constants (a,b,c), using the formula for a hexagonal system, are calculated using Eqs. (4–6).
where λ is X-ray wavelength, which is 1.54 Å, β is full width at half maximum (FWHM) of the diffraction peaks, θ is the diffraction angle, n is an integer and (hkl) are Miller indices from the JCPDS reference files. The lattice constants, a = b are obtained from the plane (100), while the plane (002) is used to obtain the lattice constant c.
Summary of the structural parameters obtained from the XRD results for all annealed Cd1−xZnxS samples grown with the different zinc chloride concentrations is presented in Table 1.
Values in brackets are standard values for the reference material. (Lattice parameters from JCPDS reference file 00-049-1302 are a = b = 4.13 Å and c = 6.71 Å and the calculated values are a = b = 4.03 and c = 6.69 Å. For JCPDS file 01-080-0006: a = b = 4.12 and c = 6.68 Å and calculated values are a = b = 4.03 and c = 6.69 Å).
As shown in the Table 1, the values of FWHM and the corresponding crystallite sizes change as ZnCl2 concentration increases in the electrolytic bath. The average crystallite size (Dav) was calculated for each ZnCl2 concentration and it ranges from 24.0 to 10.8 nm as ZnCl2 concentration increases. With no ZnCl2 in the bath, Dav is 22.1 nm. With 0.3 M ZnCl2 in the solution the average crystallite size increases to 24.0 nm. However, as more ZnCl2 (0.6 M and 0.9 M) is added into the solution the average crystallite size decreases successively to 22.2 nm and 10.7 nm respectively. This decrease in average crystallite size reflects deterioration of crystallinity of Cd1−xZnxS thin films as more Zn is incorporated into the film as a result of decrease in deposition rate of the films as will be seen later.
3.2 Compositional characterization
EDX measurements have been taken to confirm the composition of the Cd1−xZnxS thin films. Figure 4 shows two representative spectra; one for pure CdS thin film and the other for Cd1−xZnxS thin film grown at 0.6 M ZnCl2, as all the remaining samples have similar EDX spectra. From the spectra, the presence of Cd, Zn and S is observed in the Cd1−xZnxS thin films. The peaks belonging to Si, Sn and O come from the glass/FTO substrate. In addition, carbon peak comes from carbon coating used in sample preparation prior to SEM imaging. As shown in Table 2, as the concentration of ZnCl2 increases, more Zn atoms are incorporated into Cd1−xZnxS film and Zn/Cd ratio increases from 0.1 to 0.5 as ZnCl2 concentration in the bath increases from 0.3 to 0.9 M. Similarly, Zn/S ratio increases from 0.1 to 0.4. However, the percentage compositions of Cd and S decrease gradually, when more ZnCl2 is added into the electrolytic bath. In addition, due to the fact that Zn has high reduction potential as compared to Cd and S, the deposition rate of the bath decreases gradually from 14.6 to 5.0 nm/min as more ZnCl2 is added into the electrolyte. Summary of influence of ZnCl2 concentration on percentage atomic composition of each incorporated atom, deposition rate and thickness of the film is presented in Table 2.
Faraday’s equation (Eq. 7) has been used to estimate theoretical thicknesses of all the thin films [27]. The estimated thicknesses of all films at these four different zinc chloride concentrations are in the rage of 437–151 nm. The films were grown for 30 min each, for the purpose of characterization. However, for solar cell application as window/buffer materials, it is enough to grow the films for few minutes. This will reduce the thickness of the films and therefore reduce the absorbance while increasing the transmittance.
where L is theoretical thickness of the film in nm, I is the average deposition current in mA/cm2, recorded at regular interval throughout the deposition period from the computerised potentiostat, M is the molar mass of Cd1−xZnxS in grams, t is the deposition time in second, d is the density of Cd1−xZnxS in g/cm3 obtained from the JCPDS reference file used for indexing the deposition films, A is of the surface area thin film in cm2 measured using a ruler, F is Faraday’s constant and n is the number of electrons transferred in the reaction for the formation of 1 mol of CdS or CdxZn1−xS based on Eqs. (1–3).
As seen in Table 2, the thicknesses of Cd1−xZnxS thin films decrease gradually from 437 nm at 0 M ZnCl2 to 151 nm at 0.9 M ZnCl2. It is known that, thicknesses of thin films are influenced by deposition rates (nm/min), the higher rate of deposition makes the film thicker. However, due to the fact that Zn has high reduction potential as compared to Cd coupled with the high resistivity of ZnS as mentioned earlier, the deposition rate of Cd1−xZnxS thin films reduces gradually as more Zn is incorporated into the films and as more ZnCl2 is added into the electrolytic solution. This becomes evident in the drop in deposition current observed during the deposition process, thus leading to decrease in film thickness.
3.3 Raman spectroscopy
Raman spectroscopy measurement has been used to support XRD result of Cd1−xZnxS thin films. It is a fast and non-destructive tool for material identification by observing vibrational, rotational, and other low-frequency modes in a material system [28, 33]. It is also a simple way to determine the phase of the material crystalline, noncrystalline or amorphous [34]. As shown in Fig. 5, the Raman spectra of these Cd1−xZnxS thin films grown at different ZnCl2 concentrations are compared.
The measurement was taken in the wavenumber range of 200–800 cm−1 using Argon laser of wavelength 514 nm. The spectra show two dominant peaks typical of Cd1−xZnxS, and these dominant peaks belong to the longitudinal optic (LO) vibration mode. They are at wave numbers of 300 cm−1 for the first longitudinal optic (1LO) phonon and 600 cm−1 for the second longitudinal optic (2LO) phonon. There is no observed shift in the position of the 1LO peaks as ZnCl2 concentration changes. However, for the second longitudinal optic (2LO) vibration mode, there is a continuous shift in the peaks position as ZnCl2 concentration changes. For 0 M ZnCl2 (pure CdS), the peak is at a wave number of 600 cm−1 and for 0.3 M, 0.6 M and 0.9 M ZnCl2 it is found to be at 601, 602 and 606 cm−1 respectively. Furthermore, the intensity of the Raman peaks decreases as ZnCl2 increases in the electrolytic solution. These decrease in intensity of the peaks come from the incorporation of more Zn atoms in the CdS lattice and associated decrease in film thickness due to reduced deposition rate. At 0 M ZnCl2 (pure CdS), the film has the highest thickness and therefore the highest Raman peak and the smallest Raman peak is recorded when 0.9 M ZnCl2 is added in to the electrolytic solution and in which case the film thickness and deposition rate are lowest. FWHM of the Raman peaks measurement were estimated and increase in FWHM values with increase in ZnCl2 concentration in the electrolytic bath was observed in agreement with the gradual decrease in crystallite size, from XRD result, as more Zn is incorporated into the film. The highest FWHM value of 22.01 cm−1 (1LO) was recorded for the sample grown at 0.9 M ZnCl2 concentration.
3.4 Morphological properties
Figure 6 shows the surface morphology of electrodeposited Cd1−xZnxS thin film grown at different ZnCl2 concentrations. The obtained SEM images show that, all films are covered by uniformly distributed and tightly packed grains. However, the surface morphology of the films shows changes as the amount of ZnCl2 added into the electrolytic solution varies. For the sample grown with 0 M ZnCl2 (pure CdS), distinct circular shaped grains of different sizes are observed. But, when more ZnCl2 is added into the bath (i.e. as more Zn is incorporated into the film) the size of the grains become smaller and the shapes gradually change from circular to less distinctly shaped grains. The surface morphologies of Cd1−xZnxS thin films are therefore significantly influenced by the amount Zn incorporated into the film.
3.5 Optical properties
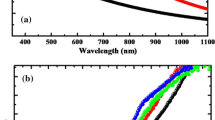
The optical properties of Cd1−xZnxS thin films deposited under different zinc chloride concentrations have been studied using UV–Vis spectroscopy in the wavelength range of 200–1000 nm and the absorbance spectra are shown in Fig. 7a. The spectra show that, pure CdS thin film has high absorbance compared to Cd1−xZnxS, which is a drawback of CdS thin film as a window/buffer material for solar cell application [35,36,37]. As Zn is incorporated into the CdS thin film, the absorbance value reduces gradually and the lowest absorbance was recorded at 0.9 M ZnCl2. This decrease in absorbance is attributed to the low thickness of CdZnS as a result of reduction in deposition rate with increasing ZnCl2 concentration in the deposition electrolyte, as already presented in Table 2. This also results in improved transmittance of the CdZnS films as more zinc is incorporated into the material. Another observation about these absorbance spectra is that the absorption edge shifts towards lower photon wavelength as more Zn is incorporated into the film, which suggests enhancement of optical band gap as seen in Fig. 7b.
To determine the energy band gap of Cd1−xZnxS thin films, the square of absorbance (Abs2) as a function of photon energy has been plotted, and the band gap energy estimated by extrapolating the straight line proportion of the graph to the photon energy axis (i.e. to Abs2 = 0). Figures 7b and 8 show that all Cd1−xZnxS thin films have higher band gaps than pure CdS thin film and also the absorption edge shifts towards higher energies as Zn-content increases. As shown in Fig. 8 the energy band gap ranges from 2.40 eV (for pure CdS) to 2.62 eV (with 0.9 M ZnCl2). The increase in concentration of ZnCl2 in the electrolytic solution leads to low deposition rate and incorporation of more Zn into the film and this results in increase in band gap of Cd1−xZnxS thin films to 2.52, 2.53 and 2.62 eV for 0.3 M, 0.6 M and 0.9 M ZnCl2 respectively. A large band gap of Cd1−xZnxS thin film (Eg = 2.62 eV) indicates an improvement in transmittance and decrease in absorbance. This agrees with the transmittance and absorbance results. It is also observed that, the improvement in band gap is almost linear with ZnCl2 concentration increase as shown in Fig. 8.
To measure the non-linear variation of optical band gap (Eg) of Cd1−xZnxS thin films over the range of composition ‘x’, modified quadratic form of the Vergard’s equation has been used as shown in Eq. (8) [5, 38].
Where Eg is experimentally measured band gap of Cd1−xZnxS thin films, Eg0 is energy band gap of CdS (2.42 eV), Eg′ is energy band gap of ZnS (3.68 eV), x is Zn composition and c is bowing parameter specifically for alloys.
The bowing parameter was calculated and it is approximately around 1.17, 1.19 and 1.17 for 0.3 M, 0.6 M and 0.9 M ZnCl2 respectively. Therefore, taking the average of these bowing parameters and using Eq. (8), the polynomial fitting of the energy band gap can be expressed as shown in Eq. (9):
Approximated value for the bowing parameter of Cd1−xZnxS thin films alloys has been reported for films grown by metal organic chemical vapour deposition (c = 0.91) and solution growth technique (c = 0.851) [5, 39]. However, to the best of our knowledge no available report on bowing parameter of Cd1−xZnxS grown by the electrodeposition method.
Figure 9 shows the transmittance spectra of the Cd1−xZnxS thin films. The transmittance increases with the incorporation of Zn into the film for the same reasons stated earlier in the case of absorbance. Unlike the absorbance spectra, increase in transmittance value is not quite proportional with the incorporation of Zn into the film rather the highest transmittance is recorded at 0.6 M ZnCl2 instead of at 0.9 M ZnCl2. However, the transmittance of the film grown with 0.9 M ZnCl2 is highest in the wavelength range 350–450 nm. This will have an advantage of improving the blue response of solar cell made with this film in comparison with the other films. As discussed in the introduction of this paper, due to its low band gap, CdS window layer has high absorption in the blue region of the solar spectrum, which results in a decrease in current density of the solar cells. However, Cd1−xZnxS thin films can resolve this issue by improving the blue response.
4 Conclusion
Two-electrode electrodeposition and characterization of Cd1−xZnxS thin films have been presented. The effect of Zn incorporation into Cd1−xZnxS thin films was studied through their structural, compositional, optical and morphological properties. All the films grown with different molar concentrations of ZnCl2 in the deposition electrolyte show polycrystalline hexagonal structure of the material. It is also observed that, as more ZnCl2 is added into the deposition solution, more Zn is incorporated into the films and intensities of the major diffraction and Raman shifts peaks of the Cd1−xZnxS films decrease proportionately as a result of low thicknesses of the films arising from reduced deposition rate. All the films are also S-rich. SEM images show uniform coverage of the film surface by Cd1−xZnxS grains and the surface morphology changes from distinct circular shaped grains to less distinct grain shapes as more Zn is incorporated into the film. Increase in ZnCl2 in the electrolytic bath results in the production of films with improved transmittance with a significant increase in the energy band gap. Maximum energy band gap of 2.62 eV was obtained with 0.9 M ZnCl2 in the electrolytic solution. Generally, the observed physico-chemical properties of these films suggest that they can effectively serve as good window/buffer materials in solar cells, with possible improvement in overall solar cell performance compared to the use of CdS.
References
S. Azizi, H.R. Dizaji, M.H. Ehsani, Structural and optical properties of Cd1–xZnxS(x = 0, 0.4, 0.8 and 1) thin films prepared using the precursor obtained from microwave irradiation processes. Optik 127, 7104 (2016)
K. Ghezali, L. Mentar, B. Boudine, A. Azizi, Electrochemical deposition of ZnS thin films and their structural, morphological and optical properties. J. Electroanal. Chem. 794, 212 (2017)
N. Gaewdang, T. Gaewdang, Investigations on chemically deposited Cd1–xZnxS thin films with low Zn content. Mater. Lett. 59, 3577 (2005)
Z. Zhou, K. Zhao, F. Huang, Optical properties of Cd1–xZnxS thin films for CuInGaSe2 solar cell application. Mater. Res. Bull. 45, 1537 (2010)
G. Kartopu, A.A. Taylor, A.J. Clayton, V. Barrioz, D.A. Lamb, S.J.C. Irvine, CdCl2 treatment related diffusion phenomena in Cd1–xZnxS/CdTe solar cells. J. Appl. Phys. 115, 104505 (2014)
S.M. Hosseinpour-Mashkani, M. Salavati-Niasari, F. Mohandes, CuInS2 nanostructures: synthesis, characterization, formation mechanism and solar cell applications. J. Ind. Eng. Chem. 20, 3800–3807 (2014)
S.M. Hosseinpour-Mashkani, M. Salavati-Niasari, F. Mohandes, K. Venkateswara-Rao, Mater. Sci. Semicond. Process. 16, 390–402 (2013)
O.I. Oladeji, L. Chow, C.S. Ferekides, V. Viswanathan, Z. Zhao, Metal/CdTe/CdS/Cd1–xZnxS/TCO/glass: a new CdTe thin film solar cell structure. Solar Energy Mater. Solar Cells 61, 203 (2000)
P. Capper, in Narrow-gap II-VI Compounds for Optoelectronic and Electromagnetic Applications, ed. by P. Capper (Chapman and Hall, New York, 1997), p. 211
Z.-Q. Qin, F.-J. Zhang, Surface decorated CdxZn1–xS cluster with CdS quantum dot as sensitizer for highly photocatalytic efficiency. Appl. Surf. Sci. 285P, 915 (2013)
B.E. McCandless, K.D. Dobson, Processing options for CdTe thin film solar cells. Sol. Energy 77, 839–856 (2004)
S. Stolyarova, M. Weinstein, Y. Nemirovsky, Growth, annealing and thermo-electrical properties of Cd1–xZnxS thin films for microbolometers. J. Cryst. Growth 310, 1674 (2008)
L. Wang, W. Wang, M. Shang, W. Yin, S. Sun, L. Zhang, Enhanced photocatalytic hydrogen evolution under visible light over Cd1–xZnxS solid solution with cubic zinc blend phase. Int. J. Hydrog. Energy 35, 19 (2010)
H. Kind, H. Yan, B. Messer, M. Law, P. Yang, Nanowire ultraviolet photodetectors and optical switches. Adv. Mater. 14, 158–160 (2002)
O. Amiri, H. Emadi, S.S.M. Hosseinpour-Mashkani, M. Sabet, M.M. Rad, RSC Adv. 4, 10990 (2014)
H.D. Dhaygude, P.P. Chikode, S.K. Shinde, N.S. Shinde, V.J. Fulari, Evaluation of the holographic parameters by electrosynthesized CdxZn1–xS (X = 0.3) thin films using double exposure digital holographic interferometry technique. Opt. Laser Technol. 88, 194 (2017)
H. Yao, H. Shen, X. Zhu, J. Jiao, J. Li, W. Wang, Influence of Cd source concentration on photo-current response property of CdxZn1–xS film prepared by chemical bath deposition. Ceram. Int. 42, 2466 (2016)
J. Hou, X. Lv, Z. Li, H. Zou, X. Zeng, Controlled synthesis of highly orientation-ordered single crystal Cd1–xZnxS nanorod array. J. Alloys Compd. 616, 97–101 (2014)
C. Tian, R. Tang, S. Hu, W. Li, L. Feng, J. Zhang, L. Wu, Comparative studies of CdZnS thin films at low zinc content prepared by vacuum evaporation and CBD. Adv. Mater. Res. 225–226, 784–788 (2011)
K. Hadasa, G. Yellaiah, M. Nagabhushanam, Optical and transport properties of Cd0.8Zn0.2S:Cu compounds prepared by modified chemical co-precipitation method. Optik 125, 6602 (2014)
S.M. Thahab, A.H.O. Alkhayat, S.M. Saleh, Influence of substrate type on the structural, optical and electrical properties of Cd1–xZnxS MSM thin films prepared by Spray Pyrolysis method. Mater. Sci. Semicond. Process 26, 49 (2014)
A.A. Ziabari, F.E. Ghodsi, Effects of the Cd:Zn:S molar ratio and heat treatment on the optical and photoluminescence properties of nanocrystalline CdZnS thin films. Mater. Sci. Semicond. Process 16, 1629 (2013)
W.S.M. Brooks, S.J.C. Irvine, V. Barrioz, A.J. Clayton, Laser beam induced current measurements of Cd1 – xZnxS/CdTe solar cells. Solar Energy Mater. Solar Cells 101, 26 (2012)
L. Zheng, Y. Lin, L. Li, G. Liang, M. Li, P. Li, Y. He, Structural properties and enhanced bandgap tunability of quaternary CdZnOS epitaxial films grown by pulsed laser deposition. J. Alloys Compd. 650, 748–752 (2015)
M.S. Hossain, M.A. Islam, M.M. Aliyu, P. Chelvanathan, T. RazykovK, K. Sopian, N. Amin, Effect of annealing on the properties of ZnxCd1–xS thin film growth by RF magnetron Co-sputtering. Energy Procedia 33, 214–222 (2013)
W. Mahmood, N.A. Shah, CdZnS thin films sublimated by closed space using mechanical mixing: a new approach. Opt. Mater. 36, 1449–1453 (2014)
S.Z. Werta, O.K. Echendu, F.B. Dejene, Z.N. Urgessa, J.R. Botha, Temperature-dependent properties of electrochemically grown CdS thin films from acetate precursor. Appl. Phys. A 124, 576 (2018)
O.K. Echendu, S.Z. Werta, F.B. Dejene, K.O. Egbo, Structural, vibrational, optical, morphological and compositional properties of CdS films prepared by a low-cost electrochemical technique. J. Alloys Compd. 778, 198 (2019)
I.M. Dharmadasa, O.K. Echendu, Electrodeposition of electronic materials for applications in macroelectronic and nanotechnology based devices. Encycl. Appl. Electrochem. (2012). https://doi.org/10.1007/978-1-4419-6996-5_37
S. Dennison, Dopant and impurity effects in electrodeposited CdS/CdTe thin film for photovoltaic applications. J. Mater. Chem. 4(1), 41–46 (1994)
S. Sain, S.K. Pradhan, Mechanochemical solid state synthesis of (Cd0.8Zn0.2)S quantum dots: microstructure and optical characterizations. J. Alloys Compd. 509, 4178 (2011)
S. Saha, S. Sain, A.K. Meikap, S.K. Pradhan, Microstructure characterization and electrical transport of nanocrystalline CdZnS quantum dots. Physica E 66, 59–66 (2015)
G. Selvan, M.P. Abubacker, A.R. Balu, Structural, optical and electrical properties of Cl-doped ternary CdZnS thin films towards optoelectronic applications. Optik 127, 4946 (2016)
R. Kulkarni, A. Pawbake, R. Waykar, A. Jadhawar, H. Borate, R. Aher, A. Bhorde, S. Nair, P. Sharma, S. Jadkar, Single crystal, high band gap CdS thin films grown by RF magnetron sputtering in argon atmosphere for solar cell applications. J. Nano-electron. Phys. 10, 03005 (2018)
A. Ashour, N. El-Kadry, S.A. Mahmoud, On the electrical and optical properties of CdS films thermally deposited by a modified source. Thin Solid Films 269, 117–120 (1995)
X. Wu, High-efficiency polycrystalline CdTe thin-film solar cells. Sol. Energy 77, 803–814 (2004)
I.O. Oladeji, L. Chow, C.S. Ferekides, V. Viswanathan, Z. Zhao, Metal/CdTe/CdS/Cd1–xZnxS/TCO/glass: a new CdTe thin film solar cell structure. Solar Energy Mater. Solar Cells 61, 203–211 (2000)
W. Xia, J.A. Welt, H. Lin, H.N. Wu, M.H. Ho, C.W. Tang, Fabrication of Cd1–xZnxS films with controllable zinc doping using a vapour zinc chloride treatment. Solar Energy Mater. Solar Cells 94, 2116 (2010)
S.V. Borse, S.D. Chavhan, R. Sharma, Growth, structural and optical properties of Cd1–xZnxS alloy thin films grown by solution growth technique (SGT). J. Alloys Compd. 436, 414 (2007)
Acknowledgements
The principal author would like to thank National Research Fund, South Africa and Dire Dawa University, Ethiopia for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Werta, S.Z., Echendu, O.K. & Dejene, F.B. Physico-chemical studies of Cd1−xZnxS thin films produced by simple two-electrode electrodeposition system for solar cell application. J Mater Sci: Mater Electron 30, 6201–6211 (2019). https://doi.org/10.1007/s10854-019-00922-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00922-z