Abstract
TiO2 nanoparticles (NP) at top of TiO2 nanotube (TiO2 NP@TiO2NT) double-layered architecture is constructed to combine the advantages of TiO2 NP and TiO2 NT together. This composite TiO2 NP@TiO2NT architecture as photoanode possesses a larger surface area for more QDs loading, and highly tubular structure for electron swift transport. Based on this architecture, CdSe/CdS quantum dots (QDs) have been successfully synthesized by successive ionic layer adsorption reaction (SILAR) method for quantum dots-sensitized solar cell application. The photovoltaic performance of QDSSCs based on TiO2 NP@TiO2 NT have been investigated in contrast with bare TiO2 NP and bare TiO2 NT architectures with almost the same thickness. The results show that the power conversion efficiency (PCE) of QDSSCs could be enhanced using TiO2 NP@TiO2 NT and improved to 3.26%, which is 80% and 38% higher than QDSSCs based on bare TiO2 NT and bare TiO2 NP, respectively. The BET surface area, UV–vis absorption spectra, and incident photon to current conversion efficiency (IPCE) measurements results show the evidence that the TiO2 NP@TiO2 NT can combine advantages of TiO2 NP and TiO2 NT structures together and lead to a higher light harvesting efficiency, electron collecting efficiency, and efficient electron transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Quantum dots-sensitized solar cells (QDSSCs) based on nanocrystalline TiO2 have presented power conversion efficiency (PCE) over 10% according to the very recent literatures [1,2,3], which is now becoming one of the most conspicuous candidate for new generation of solar cell due to their simplicity of the synthetic procedure, tunability of light absorption, and high theoretical conversion efficiency [4, 5]. A typical QDSSC consisted of QDs sensitized TiO2 layer which is constructed on conductive surface of conducting glass (such as FTO or ITO conducting glass) as the photoanode, an electrolyte solution consisted of S2−/\({\text{S}}_{x}^{2-}\) redox, and a counter electrode [6, 7]. Once illuminated by light, the QDs are excited and charges separation occurs via injection of electrons from QDs into the conduction band of TiO2 [8,9,10]. Then, electrons are transported to the external circuit through network of TiO2 [11, 12]. Hence, it is believed that the photoanode is a key component, playing a fundamental role for performance of QDSSCs in light harvesting, charge generation, and charge transport [13,14,15].
In the past years, researchers keep developing new strategies to design photoanode with purpose to improve the photovoltaic performance of QDSSCs. Xinhua Zhong’s group developed a solar paint method to fabricate Zn–Cu–In–Se QDSSC based on TiO2 matrix, and obtained an average power conversion efficiency of 4.13% [16]. Employing heterojunctions seems one of popular methods to improve performance of QDSSCs. Piyush Kar et al used mixed phase of TiO2 nanotubes with Cu, CuPt, and Pt nanoparticles to adjust the interfacial band alignment and visible light photoelectrochemical activity [17]. Jian Tian and Hongzhi Cui’s group achieved a highly efficient full solar spectrum utilization employing Ag2S quantum dot/TiO2 nanobelt heterostrucures [18]. Based on hierarchical TiO2 sphere, Nattha Buatong et al fabricated CuInTeSe QDSSC, and achieved a power conversion efficiency over 3.7% [19]. P.S. Patil et al synthesized heterostrucures TiO2/PbS/ZnS solar cells, and acquired an enhanced photovoltaic performance by a wet chemical route [20].
From the development on design of photoanode, it can be seen that the commonly materials function as supporting architectures in QDSSCs is TiO2. Therefore, it is reasonable to believe that appropriate structure of TiO2 will help to improve photovoltaic performance of QDSSCs. For now, types of TiO2 structures including TiO2 nanoparticles, nanowires, nanotubes, microspheres, and hollow cubic structure have been investigated in QDSSCs [21,22,23,24,25,26,27]. TiO2 nanoparticles are most commonly used for QDSSCs due to large surface area, however, bad electron transport and too many recombination sites limit the improvement of photovoltaic performance. Instead, highly ordered TiO2 architecture can enhance separating and delivering electrons efficient [27,28,29,30]. But power conversion efficiency of QDSSCs based on ordered TiO2 nanostrucure still cannot catch up with TiO2 nanoparticles because of the lower surface area for QDs loading.
It is well known that ideal supporting TiO2 architectures should possess large surface area, high light harvesting efficiency, and rapid electron transport capability [31, 32]. From this point of view, not any single structure of TiO2 seems can satisfy these properties at the same time. Therefore, double-layered structures have been designed in QDSSCs. Fuzhi Huang et al reported a double-layered structure of TiO2 beads on top of TiO2 nanoparticles to enhance the light harvesting efficiency [33]. Our previous work reported that TiO2 nanowire/nanotube double-layered architecture can efficiently enhance electron transport and QDs loading, finally improving photovoltaic performance of QDSSCs [34]. Inspired by these research work, we proposed to build a double-layered TiO2 architecture consisted of TiO2 nanoparticles (NP) as top-layer and highly ordered TiO2 nanotubes (NT) as under-layer. This type of TiO2 NP@TiO2 NT double-layered structure may significantly improve photovoltaic performance of QDSSCs due to its synergic functions, such as the relatively large surface area of TiO2 NP for QDs loading, swift electron transport in highly ordered TiO2 NT. Based on this TiO2 NP@TiO2 NT architecture, the CdSe/CdS QDSSCs are fabricated and an improved photovoltaic performance is achieved, pointing out the building of TiO2 NP@TiO2 NT structure is a potential strategy for development of high efficient QDSSCs.
2 Experimental
2.1 Chemicals
TiO2 nanoparticles with average size around 25 nm (P25, commercial), titanium foil in 99.6% purity, and fluorine doped tin oxide (FTO) conducting glass were purchased from Opvtech Co. Ltd. Other chemical reagents including ammonium fluoride, ethylene glycol, hydrogen peroxide (30 wt%), cadmium nitrate, selenium powder, sodium borohydride, ethylcellulose, terpinol, sodium sulfide, and sulfur powder were purchased from Aladdin Co. Ltd., which can be used directly without any purification.
2.2 Construction of TiO2 NP@TiO2 NT architecture
The free-standing TiO2 NT film was synthesized by two-step anodization process according to Chen and Xu [35]. In general, the Ti foil in size of 2.0 cm × 1.5 cm as anode was firstly anodized with 50 V for 8 h in ethylene glycol electrolyte with graphite sheet in size of 2.0 cm × 1.5 cm as cathode. Then the first anodized product was heated at 450 °C for 1 h. After heating treatment, a second anodization process was applied to the product with bias of 12 V for 10 h. Following this step, the sample was transferred into 10 wt% H2O2 aqueous solution for 12 h of immersion and finally got the free-standing TiO2 nanotube (NT) film.
The TiO2 NT film which was further cut into square piece in size of 0.16 cm2 was adhered to FTO glass using viscous paste consisted of 0.5 g ethylcellulose, 10 mL terpinol, 3 mL ethanol, and 1 mL butyl titanate as adhesive to form initial TiO2 NT photoanode. Then TiO2 nanoparticles (NP) paste consisted of 5 g TiO2 nanoparticles powders, 0.5 g ethylcellulose, 10 mL terpinol, and 3 mL ethanol, was doctor-bladed onto the top of TiO2 NT film forming double-layered TiO2 NP@TiO2 NT photoanode. After drying in ambient, the photoanode was heated in muffle furnace at 450 °C for 2 h to eliminate the organic residuals. Other architectures including TiO2 NP and TiO2 NT photoanodes with almost same thickness to the TiO2 NP@TiO2 NT were also prepared for comparison of I–V performance in the same condition (the thickness of these architectures is around 10 µm, see Fig. S1 in supplementary materials).
2.3 Sensitization of CdSe/CdS on TiO2 architectures
Successive ionic layer adsorption reaction (SILAR) method was employed to conduct the sensitization of CdSe/CdS QDs on TiO2 architectures. Initially, 8 SILAR cycles of CdS QDs were sensitized on TiO2 photoanode by dipping TiO2 photoanode in 0.1 M cadmium nitrate solution and 0.1 M sodium sulfide solution in sequence according to our previous work [29]. Then 9 SILAR cycles of CdSe QDs were sensitized by dipping CdS/TiO2 photoanodes in 0.1 M cadmium nitrate solution and 0.1 M NaSeH4 solution in sequence to form CdSe/CdS QDs sensitized TiO2 architectures including TiO2 NT, TiO2 NP, and double-layered TiO2 NP@TiO2 NT.
2.4 Counter electrode and QDSSCs assembly
Cu2S/FTO was used as counter electrode for QDSSCs which was prepared according to previous literature [36]. The SEM of Cu2S/FTO counter electrode is shown in Fig. S2 (See supplementary materials). The photoanode and counter electrode were assembled together by dropping one drop of polysulfide electrolyte consisted of 1 M sulfur and 1 M sodium sulfide in water/methanol (1:1 in volume) solution for open I–V test. We prepared CdSe/CdS QDSSCs based on TiO2 NP@TiO2 NT, TiO2 NT, and TiO2 NP architectures with almost the same thickness for comparative investigation of structure effect on solar cell performance.
3 Characterization
Quanta 450 FEG scanning electron microscopy (SEM) and Tecnai G2 F20 transmission electron microscope (TEM) which equipped with an energy dispersive X-ray spectrometer (EDS) were employed to record morphology of the prepared products. For crystal phase characterization, D/MAX-2400 X-ray diffractometer was used to analyze the crystalline phase of the samples. The optical absorption properties of the photoanodes were recorded by a U-3900H UV–vis spectrophotometer which is equipped with integrating sphere attachment for diffuse reflection measurement. The Nitrogen adsorption–desorption isotherm and BET surface area analysis were conducted by TriStar II 3020.
The I–V properties of the QDSSCs were obtained under illumination using a solar simulator to simulate sunlight with intensity of 100 mW cm−2. Each one of the photoanodes for I–V test was covered by a mask with active area of 0.16 cm2 to guarantee the same illumination area. The incident photon to charge carrier generation efficiency (IPCE) was measured as a function of wavelength by 150 W Xe lamp coupled with a computer controlled monochromator. The electrochemical impedance spectroscopy (EIS) tests of the QDSSCs were conducted by CHI852C electrochemical workstation in dark with their open circuit voltage, from 10−1 to 10−5 Hz, to explore the charge transfer dynamics of the QDSSCs.
4 Results and discussion
Figure 1a shows surface of free-standing TiO2 nanotubes. The top surface morphology shows highly ordered tubular structure with diameter around 100 nm. The bottom view indicates that the ends of nanotubes are sealed, which is in accordance with the results reported by Chen and Xu [35], proving the successful synthesis of free-standing TiO2 NT films. The cross-sectional view of TiO2 NT in Fig. 1b shows the whole free-standing TiO2 NT remain intact and the length of TiO2 NT is around 8 µm. The inset of Fig. 1b shows clear tubular structure vertical to the horizontal level, indicating potential application of the TiO2 NT for efficient electrons transportation. Based on free-standing TiO2 nanotubes (NT), composite structure (TiO2 NP@TiO2 NT) composed of TiO2 nanoparticles and TiO2 nanotubes were further built. Figure 1c displays the top surface morphology of TiO2 NP@TiO2 NT. Apparently, the top surface of TiO2 NT is covered by ~ 30 nm nanoparticles. In addition, the aggregation of TiO2 nanoparticles in some local area can be observed in Fig. 1c, which is related to the annealing process in muffle furnace. The formation of TiO2 NP@TiO2 NT may combine good adsorption ability of TiO2 NP and excellent electrons transfer capability of TiO2 NT together, leading to enhanced photovoltaic performance in QDSSCs application.
a The top surface morphology of free-standing TiO2 NT, the inset is bottom of free-standing TiO2 NT; b the cross-sectional view of TiO2 NT, the inset is local magnified SEM; c the top surface morphology of TiO2 NP@TiO2 NT; d the top surface morphology of CdSe/CdS QDs sensitized TiO2 NP@TiO2 NT; e the cross-sectional view of CdSe/CdS/TiO2 NP@TiO2 NT; f the magnified cross-sectional view of TiO2 NT selected from CdSe/CdS/TiO2 NP@TiO2 NT
Using TiO2 NP@TiO2 NT as supporting architecture, the CdSe/CdS QDs were further deposited to form CdSe/CdS/TiO2 NP@TiO2 NT photoanode by SILAR method. Obvious change of top morphology occurred after the deposition of CdSe/CdS QDs, as shown in Fig. 1d. The nanoparticles seem to contact together leading to a more severe aggregation after sensitization of QDs than that of bare TiO2 NP@TiO2 NT. Local identifiable nanotubes beneath the TiO2 NP layer also presents a decreased diameter. The cross-sectional view of CdSe/CdS/TiO2 NP@ TiO2 NT in Fig. 1e shows clearly double layered structure. The top layer (TiO2 NP) also presents similar phenomenon of aggregation, indicating the deposition of CdSe/CdS QDs. The bottom layer SEM view of TiO2 NT from CdSe/CdS/TiO2 NP@TiO2 NT photoanode was locally magnified in Fig. 1f. Although the vertical tubular structure still preserves well, the inside of the nanotubes is deposited with many small particles, indicating the CdSe/CdS QDs can not only be sensitized on top layer TiO2 NP but also deposited into the inside of underlayer TiO2 NT.
The TiO2 NT part of TiO2 NP@TiO2 NT is given in Fig. 2a, which shows empty tubular structure with diameter around 100 nm. The tubular structure can function as high efficient electrons path ways and supporting architecture for deposition of QDs. Figure 2b shows the TEM of TiO2 NP part from TiO2 NP@TiO2 NT. Apparently, the nanoparticles with diameter around 25 nm are aggregated together forming porous networks to facilitated the adsorption of QDs. The EDS of TiO2 NP@TiO2 NT is presented in Fig. 2c, which shows that the Ti and O elements consisted of the structure, and the atomic ratio close to 1:2 between Ti and O proved the composite structure is TiO2. The morphological variation was further recorded by TEM when the CdSe/CdS QDs were deposited on the TiO2 NP@TiO2 NT forming CdSe/CdS/TiO2 NP@TiO2 NT photoanode. Figure 2d displays the TEM of TiO2 NT part from CdSe/CdS/TiO2 NP@ TiO2 NT photoanode. Obviously, many QDs are distributed into the inside of nanotube, demonstrating the TiO2 NT can adsorb QDs. Moreover, the TiO2 NP part also changed greatly due to that the QDs can be found on TiO2 NP as shown in Fig. 2e. Figure 2f presents the EDS of CdSe/CdS/TiO2 NP@TiO2 NT. Apart from Ti and O elements originated from the TiO2 NP@TiO2 NT architectures, other elements such as Cd, S, and Se can be found in this EDS. The atomic ratio of Cd: (S + Se) is close to 1:1 according the quantitative analysis of EDS, indicating the successful formation of CdSe/CdS QDs on TiO2 NP@TiO2 NT structure.
a The TEM of TiO2 NT part from TiO2 NP@TiO2 NT; b the TEM of TiO2 NP part from TiO2 NP@TiO2 NT; c the EDS of designated spot; d the TEM of TiO2 NT part from CdSe/CdS/TiO2 NP@TiO2 NT photoanode; e the TEM of TiO2 NP part from CdSe/CdS/TiO2 NP@TiO2 NT photoanode; f the EDS of designated spot from CdSe/CdS/TiO2 NP@TiO2 NT
The XRD patterns of TiO2 NP@TiO2 NT and CdSe/CdS/TiO2 NP@TiO2 NT were compared in Fig. S3 (See supplementary materials). The XRD pattern of bare TiO2 NP@ TiO2 NT shows several strong diffraction peaks which can be ascribed to the anatase TiO2 (PDF # 21-1272). Moreover, a small diffraction peak at 2θ = 39.2° belonging to the (200) plane of rutile TiO2 (PDF# 21-1276) can be discerned, because the TiO2 nanoparticles (P25) nanopowder of the composite photoanode contains rutile phases. This result evidenced our composite architecture photoanode composed of TiO2 nanoparticles (P25) and TiO2 nanotubes. After sensitization of CdSe/CdS QDs, the XRD pattern of CdSe/CdS/TiO2 NP@TiO2 NT shows other diffraction peaks. A diffraction peak around 26.5° belongs to the (001) plane of cubic CdS (PDF # 43–0985). Another two diffraction peaks around 44.3° and 52.6° respectively, can be ascribed to the (220) and (311) planes of cubic CdSe (PDF # 19–0191). The XRD patterns results further confirmed the successful formation of CdSe/CdS/TiO2 NP@TiO2 NT.
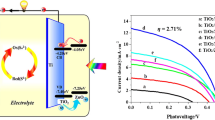
With the purpose to reveal the influence of TiO2 architectures on photovoltaic performance of QDSSCs, we built CdSe/CdS QDSSCs based on TiO2 NT, TiO2 NP and TiO2 NP@TiO2 NT architectures, respectively, and their I–V behaviors are compared in Fig. 3. The key parameters including short-circuit current density (Jsc), open voltage (Voc), fill factor (FF), and power conversion efficiency (PCE) are summarized in Table 1. The CdSe/CdS QDSSCs based on bare TiO2 NT shows a Jsc of 12.50 mA cm−2, Voc of 0.38 V, FF of 0.38, producing the lowest PCE of 1.81% among the sample solar cells. When using TiO2 NP as supporting architectures, the Jsc presented a higher value of 15.91 mA cm−2, resulting in an enhancement of PCE to 2.36%. The best photovoltaic performance of our CdSe/CdS QDSSC is achieved by using TiO2 NP@TiO2 NT double-layered structure, which its Jsc significantly improved to 21.02 mA cm−2, Voc slightly increased to 0.43 V, yielding a PCE of 3.26%.
The only difference of our solar cells is the TiO2 architectures, so it is believed that the double-layered TiO2 NP@TiO2 NT structure contributed to the significant enhancement of PCE. We believe that the double-layered TiO2 NP@TiO2 NT structure may combine the advantages of TiO2 NP and TiO2 NT together. Figure 4 illustrates the model of double-layered TiO2 NP@TiO2 NT structure. The TiO2 NP top layer may possess a larger surface area for more QDs loading, while the TiO2 NT underlayer may provide an efficient electron pathway. Once these two advantages combine together, more QDs will be involved into electrons generation under light illumination meanwhile the swift transfer of electrons will be guaranteed, leading to an enhancement of Jsc. In order to confirm our speculation, the BET surface area of double-layered TiO2 NP@TiO2 NT has been tested by N2 adsorption–desorption measurements, and the results are shown in Fig. 5. The BET surface area of bare TiO2 NT is 17.1113 m2 g−1, however, when the TiO2 NP were built at TiO2 NT forming TiO2 NP@TiO2 NT, the BET surface area can be improved to 51.9864 m2 g−1. The BET surface area of TiO2 nanoparticles (P25) as shown in Fig. S4 (See supplementary materials) is 53.6042 m2 g−1, agreeing with the literature report [16]. When the composite structure of TiO2 NP@TiO2 NT formed, the surface area is comparable to the commercial P25, confirming that the TiO2 NP@TiO2 NT double-layered structure can adsorb more CdSe/CdS QDs than TiO2 NT, more QDs loading means more electrons will be generated under illumination, which is beneficial to increase the light harvesting efficiency.
Similar inference can also be evidenced by UV–vis spectra as shown in Fig. 6. Two interesting phenomena can be observed: one is that the onset of light absorption is identical (around 660 nm) when CdSe/CdS QDs are sensitized on TiO2 NT, TiO2 NP, or TiO2 NP@TiO2 NT structure, indicating the light absorption depend on CdSe/CdS QDs; another one is that the absorbance of CdSe/CdS QDs sensitized TiO2 NP@TiO2 NT photoanode is the highest, indicating more QDs are adsorbed by TiO2 NP@TiO2 NT structure, which will facilitate photons absorption to generate more electrons.
To decipher the effect of TiO2 supporting architecture on CdSe/CdS QDSSCs, the incident photon to current conversion efficiencies (IPCE) are monitored between excitation wavelength of 300 nm and 800 nm, which are shown in Fig. 7. Comparison of these IPCE spectra, it can be found that the double-layered TiO2 NP@TiO2 NT structure yield the highest photocurrent than TiO2 NP or TiO2 NT structure. As reported previously [12], the TiO2 NT architecture exhibits better electron collecting and transport properties than the TiO2 NP architecture. In our case, the IPCE based on TiO2 NP is higher than TiO2 NT. The reason responsible for this may be the less light harvesting efficiency caused by lower BET surface area of TiO2 NT. However, it is worth noting that photocurrent significantly improved when the TiO2 NP@TiO2 NT structure was built. This results indicates that the TiO2 NP top layer compensates the weak adsorption ability of TiO2 NT, and the TiO2 NT underlayer guarantees the higher efficient of electron collecting and transport. To testified the inference that the TiO2 NP@TiO2 NT combine advantages of TiO2 nanoparticles and nanotube together, we analyzed CdSe/CdS QDSSCs based on TiO2 NT, TiO2 NP, and TiO2 NP@TiO2 NT via EIS in contrast. Figure 8 exhibits the typical Nyquist plot of the EIS results. The large semicircle represents the impedance associated with the charge transfer at the QDs/electrolyte interface [37]. Larger semicircle represents lager charge transport resistance at the QDs/electrolyte interface, indicating a better electrons collecting and transport properties of TiO2 architecture. As shown in Fig. 8, the TiO2 NT shows the best electron collecting and transport ability in comparison with TiO2 NP and TiO2 NP@TiO2 NT. However, the best photovoltaic performance is achieved by TiO2 NP@TiO2 NT. What is the reason should responsible for this phenomenon? As we discussed earlier, the bare TiO2 NT has a small surface area, even though it can provide high efficient electron transport, the small surface area cannot adsorb enough CdSe/CdS QDs to generate numbers of electrons. When TiO2 NP@TiO2 NT formed, the top TiO2 NP layer can provide a larger surface area, and meantime the high electron collecting and transport of TiO2 NT also preserved. The relationship between these factors is given by the following equation:
In which LHE is the light harvesting efficiency, Φinj is the quantum yield of the electron injection from the excited electron in the QD conduction band into the TiO2 conduction band, and ηcc is the charge collecting efficiency. The LHE is related to the QDs loading. Our TiO2 NP@TiO2 NT structure has a larger surface area than TiO2 NT, which can adsorb more QDs to enhance LHE. In addition, the better electron collecting and transport ability of TiO2 NP@TiO2 NT than TiO2 NP (See EIS in Fig. 8) improve the ηcc, finally leading to the increase of IPCE and Jsc. The increment of IPCE and Jsc will also contribute to the increase of Voc according to the equation proposed by previous literature [38]:
In which EFn is the quasi-Fermi level of the electrons in semiconductor under illumination; Eredox is the potential of the redox electrolyte; e is the positive elementary charge; kBT is the thermal energy; n is the electron concentration in conduction band of the semiconductor under illumination; n0 is the electron concentration in the dark condition. As the earlier discussion about IPCE and EIS indicated, the LHE and ηcc using TiO2 NP@TiO2 NT are better than using TiO2 NP or TiO2 NT, therefore, more electrons will be accumulated in the conduction band of TiO2 and lead to an increment of n, while the Eredox remain same because of the polysulfide electrolyte is identical. Therefore, the Voc can be enhanced using double-layered TiO2 NP@TiO2 NT as supporting architecture in CdSe/CdS QDSSCs. These phenomena were consistently supported by UV–vis, IPCE, and EIS results.
5 Conclusions
In general, TiO2 NP@TiO2 NT double-layered architecture co-sensitized by CdSe/CdS QDs was employed as photoanode in QDSSCs to enhance photovoltaic performances. The results showed that the PCE of CdSe/CdS QDSSCs based on TiO2 NP@TiO2 NT double-layered architecture can be significantly enhanced than using TiO2 NT or TiO2 NP as supporting architecture. The PCE improvement by maximum 3.26%, as compared with CdSe/CdS QDSSCs based on bare TiO2 NT or TiO2 NP architecture, should be ascribed to the increased surface area of TiO2 NP top layer for enhancement of light harvesting efficiency and highway of TiO2 NT underlayer for efficient electron transport. These results indicate that the TiO2 NP@TiO2 NT double-layered architecture will deliver insights into the design of supporting structure of TiO2 for high photovoltaic performance QDSSCs.
References
J. Du, Z. Du, J.S. Hu, Z. Pan, Q. Shen, J. Sun, D. Long, H. Dong, L. Sun, X. Zhong, L.J. Wan, J. Am. Chem. Soc. 138, 4201 (2016)
W. Wang, W. Feng, J. Du, W. Xue, L. Zhang, L. Zhao, Y. Li, X. Zhong, Adv. Mater. 30, 1705746 (2018)
S. Jiao, J. Du, Z. Du, D. Long, W. Jiang, Z. Pan, Y. Li, X. Zhong, J. Phys. Chem. Lett. 8, 559 (2017)
P.V. Kamat, J. Phys. Chem. Lett. 4, 908 (2013)
A. Badawi, Superlattices Microstruct. 90, 124 (2016)
J. Sun, H. Cuo, L. Zhao, S. Wang, J. Hu, B. Dong, Int. J. Electrom. Sci. 12, 7941 (2017)
H.K. Jun, M.A. Careem, A.K. Arof, Renew. Sustain. Energ. Rev. 22, 148 (2013)
M. Grätzel, Nature. 414, 338 (2001)
S. Ruhle, M. Shalom, A. Zaban, ChemPhysChem 11, 2290 (2010)
D.-W. Jeong, J.-Y. Kim, H.W. Seo, K.-M. Lim, M.J. Ko, T.-Y. Seong, B.S. Kim, Appl. Surf. Sci. 429, 16 (2018)
P.V. Kamat, Acc. Chem. Res. 45, 1906 (2012)
D.R. Baker, P.V. Kamat, Adv. Funct. Mater. 19, 805 (2009)
J.-Y. Liao, B.-X. Lei, H.-Y. Chen, D.-B. Kuang, C.-Y. Su, Energy Environ. Sci. 5, 5750 (2012)
W.-T. Sun, Y. Yu, H.-Y. Pan, X.-F. Gao, Q. Chen, L.-M. Peng, J. Am. Chem. Soc. 130, 1124 (2008)
R. Zhou, Q. Zhang, E. Uchaker, J. Lan, M. Yin, G. Cao, J. Mater. Chem. A. 2, 2517 (2014)
G. Shen, Z. Du, Z. Pan, J. Du, X. Zhong, ACS Omega 3, 1102 (2018)
P. Kar, Y. Zhang, N. Mahdi, U.K. Thakur, B.D. Wiltshire, R. Kisslinger, K. Shankar, Nanotechnology. 29, 014002 (2018)
X. Hu, Y. Li, J. Tian, H. Yang, H. Cui, J. Ind. Eng. Chem. 45, 189 (2017)
N. Buatong, I. Tang, W. Pon-On, Mater. Lett. 199, 41 (2017)
T.S. Bhat, S.S. Mali, A.D. Sheikh, S.D. Korade, K.K. Pawar, C.K. Hong, J.H. Kim, P.S. Patil, Opt. Mater. 73, 781 (2017)
S. Cheng, W. Fu, H. Yang, L. Zhang, J. Ma, H. Zhao, M. Sun, L. Yang, J. Phys. Chem. C 116, 2615 (2012)
K. Guo, M. Li, X. Fang, L. Bai, M. Luoshan, F. Zhang, X. Zhao, J. Power Sources. 264, 35 (2014)
L. Yu, Z. Li, Y. Liu, F. Cheng, S. Sun, Appl. Surf. Sci. 309, 255 (2014)
Y. Bai, I. Mora-Seró, F. De Angelis, J. Bisquert, P. Wang, Chem. Rev. 114, 10095 (2014)
Z. Li, L. Yu, Y. Liu, S. Sun, J. Mater. Sci. Mater. Electron. 26, 1625 (2015)
G. Veerappan, K. Zhang, S. Soman, N. Heo, J.H. Park, Sol. Energy 157, 434 (2017)
U.K. Thakur, A.M. Askar, R. Kisslinger, B.D. Wiltshire, P. Kar, K. Shankar, Nanotechnology. 28, 274001 (2017)
C. Chen, F. Li, G. Li, F. Tan, S. Li, L. Ling, J. Mater. Sci. 49, 1868 (2013)
X. Ren, L. Yu, Z. Li, H. Song, Q. Wang, Superlattices Microstruct. 113, 696 (2018)
X. Deng, Y. Wang, Z. Cui, L. Li, C. Shi, Superlattices Microstruct. 117, 283 (2018)
Y. Liu, K. Lan, A.A. Bagabas, P. Zhang, W. Gao, J. Wang, Z. Sun, J. Fan, A.A. Elzatahry, D. Zhao, Small. 12, 860 (2016)
X. Wang, J. Wang, H. Wang, J. Nanopart. Res. 16, 2458 (2014)
F. Huang, D. Chen, X.L. Zhang, R.A. Caruso, Y.-B. Cheng, Adv. Funct. Mater. 20, 1301 (2010)
Z. Li, L. Yu, Y. Liu, S. Sun, Electrochim. Acta 129, 379 (2014)
Q. Chen, D. Xu, J. Phys. Chem. C 113, 6310 (2009)
S.S. Kalanur, S.Y. Chae, O.S. Joo, Electrochim. Acta 103, 91 (2013)
Y. Choi, M. Seol, W. Kim, K. Yong, J. Phys. Chem. C 118, 5664 (2014)
A. Zaban, M. Greenshtein, J. Bisquert, ChemPhysChem. 4, 859 (2003)
Acknowledgements
This work has been financially supported by the University Research Project of Gansu Province [No. 2017A-089, 2018A-091], the Surface Project of Key Laboratory of Hexi Corridor Resources Utilization of Gansu Province [No. XZ1604], and the Hexi University Principle Fund of Scientific Innovation and Application [No. XZ2017010].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Yu, L., Song, H. et al. Construction of TiO2 NP@TiO2 NT double-layered structural photoanode to enhance photovoltaic performance of CdSe/CdS quantum dots sensitized solar cells. J Mater Sci: Mater Electron 29, 18059–18066 (2018). https://doi.org/10.1007/s10854-018-9915-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9915-1