Abstract
The 0.2Li2ZrO3–0.8MgO (Li2Mg4ZrO7) ceramics were prepared by conventional solid-state reaction method. The effects of lithium fluoride (LiF) addition on the microstructure, sintering temperature and microwave dielectric properties of Li2Mg4ZrO7 ceramics were investigated. The experimental results demonstrated a typical single phase existed in Li2Mg4ZrO7–LiF ceramics, in which LiF was proved to be an effective sintering aid for the Li2Mg4ZrO7 ceramic. LiF obviously lowed the optimum sintering temperature of Li2Mg4ZrO7 ceramic from 1500 to 1050 °C and improved the sintering ability of the matrix. In this paper, the microwave dielectric properties were strongly dependent on the amount of LiF additions and the sintering temperature. Optimum microwave dielectric properties (εr = 12.95, Q·f = 180,011 GHz and τf =− 23.51 ppm/°C) were obtained in Li2Mg4ZrO7-4 wt% LiF ceramics sintered at 1050 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microwave dielectric ceramics or even thin films are widely used in various devices, including resonator, duplexer and multiplexer for wireless communication [1,2,3]. To meet the requirement of the microwave devices, high quality factor (Q·f), a near-zero temperature coefficient of resonant frequency (τf) and large dielectric permittivity (εr) must be achieved [4, 5]. Additionally, these materials are required to be sintered at the lower temperatures because they could be cofired with benign conductors such as silver and aluminium [6]. However, most of the commercial dielectric ceramics do not satisfy the stringent and increasing demands of microwave communication systems because of their high sintering temperature. Therefore, the search for new low-temperature sintering microwave dielectric ceramics has attracted more and more attention.
In our previous work, 0.2Li2ZrO3 + 0.8MgO (Li2Mg4ZrO7) ceramic with cubic structure was reported by Bi et al., which possessed suitable microwave dielectric properties with εr = 12.65, Q·f = 165,924 GHz and τf =− 34.66 ppm/°C in the microwave range [7]. Because of the excellent microwave dielectric properties, the Li2Mg4ZrO7 ceramics were promising candidates to be used widely. However, for the practical applications for Li2Mg4ZrO7 ceramics, key challenges remained, such as the higher sintering temperatures, the higher dielectric losses and the lower dielectric constants. Therefore, it was essential to develop low-temperature sintering Li2Mg4ZrO7 based ceramics for the commercial applications. To address the above issues, there are three methods for lowering the sintering temperature: (1) adding additions with low melting point, such as LiF, Li2CO3 and glass [8,9,10,11,12]; (2) reducing the particle sizes of starting materials for chemical processing [13]; (3) forming solid solutions through ionic substitution [14,15,16]. Compared with other conventional methods, adding LiF addition was a facile strategy for lowering the sintering temperature of microwave dielectric materials, which has developed rapidly in recent years [17,18,19]. For instance, Zhang et al. investigated that CaMg0.9Zn0.1Si2O6 ceramic doped with 0.6 wt% LiF could be sintered in the temperature range of 900–975 °C and exhibited a relatively high quality factor of ~ 70,000 GHz [17]. Moreover, Tong et al. reported that the Ca[(Li0.33Nb0.67)0.9Ti0.1]O3−δ-20 wt% LiF ceramics possessed suitable microwave dielectric properties (εr = 21.3, Q·f = 20,450 GHz and τf =− 18 ppm/°C) [18]. Optimum microwave dielectric properties with εr = 22.4, Q·f = 110,000 GHz and τf = 3.2 ppm/°C could be obtained for the 0.9Li2TiO3 + 0.1LiF ceramic after sintering at 1100 °C [19]. However, research focusing on the effects of LiF additions on sintering behaviors and microwave dielectric properties of Li2Mg4ZrO7 ceramics was not found. Therefore, an investigation was made to lower sintering temperature of Li2Mg4ZrO7 ceramics in this work.
In the present work, Li2Mg4ZrO7-x wt% LiF (x = 0–5) ceramics were successfully prepared by conventional solid-state reaction. Different weight percentages of lithium fluoride were used as sintering aids. Scanning electron microscopy (SEM), X-ray diffraction (XRD) and microwave dielectric resonance measurement techniques were used to analyze the Li2Mg4ZrO7-x wt% LiF (x = 0–5) ceramics. The relationships between microwave dielectric properties and LiF contents were also investigated.
2 Experimental procedure
Li2Mg4ZrO7 ceramics were prepared by the conventional solid-state reaction, using high purity MgO, Li2CO3 and ZrO2 phases, which were mixed with mole ratio 4:1:1 and ball-milled with ethanol for 24 h. The powders were then dried and calcined at 1050 °C for 2 h. The calcined powders were re-ball-milled with 0–5 wt% LiF additions for 24 h, dried, and 8 wt% polyvinyl alcohol was added for granulation. The powder mixtures were pressed into 10 mm diameter disks and 6 mm height at a pressure of about 200 MPa. Eventually, these samples were sintered at 900–1600 °C for 6 h.
The crystal structures of Li2Mg4ZrO7-x wt% LiF (x = 0–5) were identified by an X-ray diffractometer (Model D/MAX-B, Rigaku Co., Japan). To examine the morphology, the surfaces were examined by scanning electron microscopy (FeSEM Quanta 250, FEI Co., USA). The dielectric constants at microwave frequencies were measured by the Hakki-Coleman dielectric resonator method in the TE011 mode using a network analyzer (N5234A, Agilent Co., USA) [20], and the unloaded quality factors were measured using TE01d mode by the cavity method [21]. The τf value was measured according to τf = Δf/(f0ΔT), where f0 was the resonant frequency at 25 °C.
3 Results and discussions
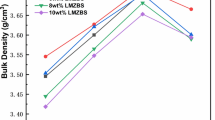
Figure 1 illustrated the variation in the apparent densities of Li2Mg4ZrO7-x wt% LiF (x = 0–5) ceramics as a function of sintering temperature. The saturated value (~ 3.52 g/cm3) of Li2Mg4ZrO7 ceramics could be obtained at 1500 °C, which was similar to the optimum result reported by Bi et al. [7]. Generally, LiF could decrease the sintering temperature and improve the sintering ability of the ceramics. When the samples were added with 1–2 wt% LiF, the apparent densities exhibited lower values compared with that of Li2Mg4ZrO7-x (x = 3–5 wt% LiF) samples. Relatively high value of the Li2Mg4ZrO7-x wt% LiF (x = 1–2) samples was ~ 2.93 g/cm3, which demonstrated that the 1–2 wt% LiF additions were insufficient to improve the densification procedure of Li2Mg4ZrO7 ceramics. The apparent density of Li2Mg4ZrO7-3 wt% LiF ceramics reached to 3.50 g/cm3 at 950 °C, which was close to that of the matrix sintered at 1500 °C. Hence, adding appropriate amount of LiF addition was one of the effective methods to decrease the sintering temperatures of the Li2Mg4ZrO7 system. Similarly, the maximum densities of the samples with 4 and 5 wt% LiF additions sintered at 950 °C were 3.60 and 3.69 g/cm3, respectively. Thereafter the maximum values of the Li2Mg4ZrO7-x wt% LiF (x = 3–5) samples the kept stable with the increasing sintering temperature. Therefore, the Li2Mg4ZrO7-x wt% LiF (x = 3–5) ceramics with relatively high density could be obtained in the temperature region of 950–1100 °C. In conclusion, adding appropriate amount of LiF additions was a facile strategy to improve the sintering characteristics of the matrix.
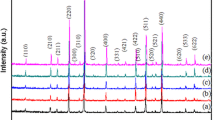
Rietveld refinement of Li2Mg4ZrO7 ceramic sintered at 1500 °C was performed by FullProf suite and the refinement plot of the composition was shown in Fig. 2. Rietveld discrepancy factors Rp and Rwp were 19.0 and 14.3%, which confirmed the validity of matching models. The ceramic was completely full of cubic phase and the lattice parameters of Li2Mg4ZrO7 were refined as V = 77.37 Å3 and a = b = c = 4.26 Å. The schematic crystal structure of cubic structured Li2Mg4ZrO7 was shown in the inset of Fig. 2. In addition, the bond length of Li/Mg/Zr–O was calculated to be 2.13 Å. It could be observed that three kinds of cations occupied the same atomic positions and were connected with other six oxygen ions.
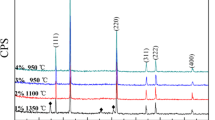
Figure 3 showed the XRD patterns of Li2Mg4ZrO7-x wt% LiF (x = 1–5) ceramics sintered at 1050 °C. For the structure information of Li2Mg4ZrO7-x wt% LiF (x = 1–5) ceramics, the corresponding intensity peaks for the ceramics were recorded. Beside the peaks of substrates, no obvious peaks belonging to LiF were found owing to the small amount of LiF in the system. All the obtained patterns were characteristic of a cubic structure with space group Fm-3m. As LiF content increased, all peaks were indexed as a single phase and identified by the JCPDS card (No. 45-0946). Hence, it could be considered that the continuous solid solutions between Li2Mg4ZrO7 and LiF were formed in this work. Typical SEM micrographs of Li2Mg4ZrO7-x wt% LiF (x = 1–5) ceramics were illustrated in Fig. 4a–e. The surface morphology of samples, illustrated in this figure, revealed the porous structure. The porosity of Li2Mg4ZrO7-x wt% LiF (x = 1–5) ceramics decreased with the increasing LiF additions. The relatively dense microstructures could be formed for Li2Mg4ZrO7 samples doped with 3–5 wt% LiF, which indicated that the appropriate amount of LiF additions improved the sintering behavior of Li2Mg4ZrO7 ceramics.
The dielectric constants for Li2Mg4ZrO7 ceramics with different amount of LiF additions were presented in Fig. 5. It was noticeable that the dielectric constants of Li2Mg4ZrO7 ceramics increased with the increasing sintering temperature, which showed a similar trend to that of the densities. The correlation between the sintering temperatures and the εr values of Li2Mg4ZrO7-x wt% LiF (x = 1–5) ceramics also presented the similar tendency with that of the densities within the temperature range of 900–1150 °C. With x value increasing from 1 to 5, the dielectric constants showed an upward tendency and were higher than that of matrix for x > 3, which illustrated that LiF could enhance the εr value by improving sintering character. Generally, the dielectric constant is influenced by the density and the secondary phase [22]. The XRD patterns in Fig. 3 indicated the pure phase of Li2Mg4ZrO7-x wt% LiF (x = 1–5) ceramics. Therefore, the dielectric constants of the sintered samples were closely related to the apparent densities in this work. However, as the sintering temperature increasing from 950 to 1150 °C, there was no obvious change in the dielectric constants of Li2Mg4ZrO7-x wt% LiF (x = 3–5) ceramics at a given LiF content, which proved that the sintering temperature had little effect on the dielectric constant when samples were in high densification [23].
The Q·f values of Li2Mg4ZrO7-x wt% LiF (x = 0–5) ceramics were shown in Fig. 6. The quality factors are affected by intrinsic losses and extrinsic losses. The extrinsic losses are dominated by densification, porosity, second phases and microcracks, while the phase composition or internal vibrations will be contributed to the intrinsic loss [24]. In this work, the extrinsic losses acted as critical factors on the Q·f values at a lower temperatures range. The quality factors of Li2Mg4ZrO7-x wt% LiF (x = 3–5) ceramics were higher than that of Li2Mg4ZrO7-x (x = 1–2 wt% LiF) samples. For instance, the Q·f values of 1 wt% LiF-doped Li2Mg4ZrO7 ceramics increased from 21,839 GHz at 1000 °C to 32,709 GHz at 1150 °C, which was correlated with the increasing apparent densities shown in Fig. 1, while the quality factors of Li2Mg4ZrO7-4 wt% LiF ceramics significantly increased with the sintering temperature increasing from 900 to 1100 °C and reached a maximum value of 180,011 GHz at 1050 °C, which was higher than the optimum value (~ 165,900 GHz) of the matrix sintered at 1500 °C. Hence, the appropriate LiF additives could make the microwave quality factors higher by improving sintering characteristics of Li2Mg4ZrO7 ceramics.
Furthermore, the microwave dielectric properties of Li2Mg4ZrO7-x (x = 0–5 wt% LiF) samples sintered at their optimum sintering temperatures were exhibited in Table 1. Because of the increase in density, a rapidly increasing trend of εr values occurred when LiF content increasing from 1 to 5 wt%. The samples showed a higher εr values than that of matrix for x > 3. With the increase of LiF content, the quality factor first increased from 31,606 to 180,011 GHz at x = 4 and then decreased slightly to 164,835 GHz at x = 5 because of the variation of the density. With the additions of LiF increasing from 1 to 2 wt%, the τf values increased from − 27.53 to − 19.12 ppm/°C, implying that the temperature stability of ceramics was sensitive to the apparent densities. After that, the τf values initially increased to − 23.51 ppm/°C and then decreased with the increasing LiF content. All the τf values of Li2Mg4ZrO7-x (x = 1–5 wt% LiF) samples sintered at 1050 °C were higher than that (− 34.7 ppm/°C) of matrix sintered at 1500 °C, which illustrated that LiF could make the ceramics more stable by improving the sintering ability of Li2Mg4ZrO7 ceramics. Typically, Li2Mg4ZrO7-4 wt% LiF ceramics sintered at 1050 °C possessed a single phase with excellent microwave dielectric properties of εr = 12.95, Q·f = 180,011 GHz and τf = − 23.51 ppm/°C.
4 Conclusions
Li2Mg4ZrO7-x wt% LiF (x = 0–5) ceramics were successfully prepared by conventional solid-state reaction. Our work provided a facile strategy for lowing the sintering temperature of Li2Mg4ZrO7 ceramics. Appropriate amount of LiF additions could effectively improve the sintering characteristics of the matrix. The εr values had a similar tendency with the densities when the LiF content and the sintering temperature increased. The Li2Mg4ZrO7 ceramic with 4 wt% LiF sintered at 1050 °C exhibited a maximum Q·f value because of its dense microstructure. Changes in the microwave dielectric properties were caused by the variation of the amount of LiF addition and the sintering temperature. Li2Mg4ZrO7-4 wt% LiF ceramic sintered at 1050 °C exhibited a single phase with microwave dielectric properties of εr = 12.95, Q·f = 180,011 GHz and τf = − 23.51 ppm/°C, which could be applied for potential applications.
References
I.M. Reaney, D. Iddles, J. Am. Ceram. Soc. 89, 2063 (2006)
T.A. Vanderah, Science 298, 1182 (2002)
C.H. Yang, Y.J. Han, X.S. Sun et al., Ceram. Int. 44, 6330 (2018)
H. Ohsato, Ceram. Int. 38, 141 (2012)
R.J. Cava, J. Mater. Chem. 11, 54 (2001)
M.T. Sebastian, H. Jantunen, Int. Mater. Rev. 53, 57 (2008)
J.X. Bi, C.F. Xing, C.H. Yang et al., J. Eur. Ceram. Soc. 38, 3840 (2018)
T. Byeon, H.S. Park, H. Shin et al., J. Korean Ceram. Soc. 47, 325 (2010)
K.S. Kim, H.S. Jang, H. Shin et al., J. Korean Ceram. Soc. 46, 323 (2009)
H. Zhuang, Z.X. Yue, F. Zhao et al., J. Am. Ceram. Soc. 91, 3275 (2008)
H.Z. Zuo, X.L. Tang, H. Guo et al., Ceram. Int. 43, 13913 (2017)
M. He, H.W. Zhang, J. Mater. Sci. 24, 3303 (2013)
H.P. Wang, S.Q. Xu, B. Zhang et al., Mater. Res. Bull. 44, 619 (2009)
M. Thirumal, I.N. Jawahar, K.P. Surendiran et al., Mater. Res. Bull. 37, 185 (2002)
J. Pei, Z.X. Yue, F. Zhao et al., J. Alloys Compd. 459, 390 (2008)
Z.X. Fang, B. Tang, Y. Yuan et al., J. Am. Ceram. Soc. 101, 252 (2018)
J. Zhang, Y.Y. zhou, Z.X. Yue, Ceram. Int. 39, 2051 (2013)
J.X. Tong, Q.L. Zhang, H. Yang et al., Mater. Lett. 59, 3252 (2015)
J.J. Bian, Y.M. Ding, Ceram. Int. 40, 3595 (2014)
B.W. Hakki, P.D. Coleman, IRE Trans. Microw. Theory Tech. 8, 402 (1960)
W.E. Courtney, IEEE Trans. Microw. Theory Tech. 18, 476 (1970)
Y. Zhao, P. Zhang, J. Alloys Compd. 662, 455 (2016)
J. Li, Y. Han, T. Qiu, C. Jin, Mater. Res. Bull. 47, 2375 (2012)
C.L. Huang, S.S. Liu, Jpn. J. Appl. Phys. 46, 283 (2007)
Acknowledgements
This work was supported by the National Natural Science Foundation (No. 51472108) and project funded by China Postdoctoral Science Foundation (2017M612341). The authors are thankful to the help of Professor Zhenxing Yue and postdoctoral Jie Zhang on the measurement of microwave properties in Tsinghua University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xing, C.F., Liu, F.L., Bi, J.X. et al. Low-temperature sintering and microwave dielectric properties of LiF-doped 0.2Li2ZrO3–0.8MgO ceramics. J Mater Sci: Mater Electron 29, 13746–13750 (2018). https://doi.org/10.1007/s10854-018-9505-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9505-2