Abstract
Microwave-assisted co-precipitation method was adopted to analyze the effect of polyethylene glycol (PEG) and urea concentrations on the properties of cobalt ferrite nanoparticles (NPs). The average crystallite size of single phase cubic spinel cobalt ferrite NPs was controlled within 10–14 nm with the effect of PEG, urea and the combination of them. The transmission electron micrographs revealed that the morphology of cobalt ferrites was not significantly influenced by the different concentration of capping agents but almost uniform morphology with nearly narrow size distribution was obtained. The interaction of PEG and urea molecules on the surface of nanoparticles was mediated through –OH hydroxyl group affected the crystal growth rate. The possible interaction mechanism was proposed with the help of IR vibrational spectra. All the samples exhibited ferromagnetism at room temperature and it was found that the capping agents showed an effect on the magnetic properties. The maximum saturation magnetization of 58 emu/g was achieved when the urea of 60 mg was used and the maximum coercivity of 311 Oe was attained when the mixture of PEG (40 mg) and urea (20 mg) were used. Ultrafine and hydrophilic cobalt ferrite NPs that showed appreciable magnetic properties obtained in the present experimental procedure would be of great interest in various biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Realization of spinel ferrite nanoparticles of uniform morphology with nearly narrow size distribution is of increasing interest among the researchers owing to their demand in biomedical, drug delivery, hyperthermia, magnetic sensors, magnetic refrigerants, highly stable ferrofluids, etc. [1,2,3,4]. Though various spinel ferrites have been identified for the applications the special attention is being given to cobalt ferrite nanoparticles (NPs). It shows moderate saturation magnetization, higher coercivity, large magnetocrystalline anisotropy constant and chemical stability [5]. The different physical and chemical methods have been proposed for the synthesis of cobalt ferrite nanoparticles where the co-precipitation method is widely being used due to their simple, inexpensive and easy approach than others [6, 7]. At the same time, microwave assisted wet chemical route is reported to be the efficient method to control the particle size distribution with almost uniform morphology [8, 9]. Microwave irradiation is a powerful heating source which improves the reaction rate, leading to short reaction time, uniform nucleation and crystal growth rates compared to conventional heating systems [8, 10]. Recently, we have found that the experimental parameters, reaction temperature [6], reaction time, and the concentration of alkaline solution plays a major role in controlling the particle size distribution of cobalt ferrite NPs synthesized using co-precipitation and microwave assisted co-precipitation method where the effect of microwave irradiation on the properties is discussed [11].
In this report, we are interested to study the effect of capping agents on the properties of cobalt ferrite NPs. The extensive work has been found on the functionalization of magnetite nanoparticles to study the effect of polyethylene glycol (PEG), and oleic acid concentration on the particle formation, size distribution, shape, magnetic properties and their stability [1, 2, 12]. Similarly, carboxylic acids and PEG assisted synthesis of nickel ferrites have been reported [13]. Also, we found some articles that report on the effect of different surfactant, capping agents on the properties of cobalt ferrite NPs. Abbasi et al. reported the synthesis of cobalt ferrite nanoparticles through a hydrothermal method using bis-(2-hydroxyl-1-naphthaldehyde)-butanediamine Schiff-base ligand as a capping agent and sodium dodecyl sulfate as a surfactant [14]. Andrade et al. studied the effect of oleic acid and oleic acid plus fucan coating on the properties of superparamagnetic cobalt ferrite nanoparticles [3]. Effect of oleic acid concentration on the particle size, magnetic properties of cobalt ferrite NPs and the interaction between them were discussed by Jovanović et al. [15]. Esir et al. synthesized cobalt ferrite NPs using PEG assisted route and their temperature dependent magnetic properties were reported [16]. Similarly, other research groups discussed the effect of glucose, lactose, starch [17] and gelatin as capping agents on the particle size, morphology and magnetic properties of cobalt ferrite nanoparticles [18].
However, to the best of our knowledge no reports that discuss the effect of PEG and urea concentrations on the structural and magnetic properties of cobalt ferrite nanoparticles. The available reports either discuss the effect of capping agent with the single concentration or no such elaborated investigations on the properties of cobalt ferrites are found. PEG and urea are selected as capping agents for the study due to their high water soluble and environmentally friendly nature to functionalize the cobalt ferrite NPs into hydrophilic magnetic NPs [1, 2, 19]. Hence, the present article is devoted to study and analyze the effect of the different combination of capping agents (PEG and urea) on the control over the particle size, its distribution, morphology and magnetic properties of cobalt ferrite NPs synthesized using microwave assisted co-precipitation method. In addition, the possible interaction mechanism between the nanoparticles and capping agents is proposed.
2 Materials and methods
Cobalt nitrate hexahydrate (Co (NO3)2·6H2O) 98% + A.C, iron nitrate nonahydrate (Fe (NO3)3·9H2O) 98% and sodium hydroxide (NaOH) pellets ≥98% ACS grade were used as the starting materials. Urea and polyethylene glycol (PEG) with a molecular weight of 800 g/mol were used as capping agents. The chemicals were purchased from Sigma-Aldrich and were used without further purification. Millipore water was used as a solvent for the synthesis.
3 Experimental procedure
The cobalt ferrite nanoparticles (NPs) were synthesized using microwave assisted co-precipitation method. The procedure for the synthesis of cobalt ferrite NPs is normally comprised of the following steps. The first process is the preparation of nitrate solutions. 20 ml of (1 M) aqueous cobalt nitrate and 20 ml of (2 M) aqueous iron nitrate solutions were prepared, separately. The aqueous nitrate solutions were then mixed and stirred at 500 rpm for 20 min. To analyze the effect of capping agents on the properties of cobalt ferrite NPs, the different weight ratios of PEG and urea were selected. The desired amount (in milligrams) of the capping agents was dissolved in the above solution mixture at room temperature and stirred vigorously for half an hour. Then, 2.52 M of 20 ml of sodium hydroxide solution was added drop by drop while stirring. The brown precipitates thus obtained were magnetically stirred for 15–20 min and then the precipitated suspension was transferred to the ceramic crucible of the volume of 100 ml. Subsequently, the precipitated brown suspension was exposed to the microwave irradiation for 7 min. The domestic microwave oven model SOMELA (Mirage 1700 DM), with a power of 700 W was used as a source of irradiation. After the microwave irradiation, the brown suspension was converted to black and was allowed to cool down naturally. The precipitated suspension was washed with Millipore water and acetone 5 times, centrifuged at 8000 rpm for 5 min and dried at 100 °C for 3 h. In this study, the ratios of PEG and urea were used as 0:0 (CF0), 0:60 (CF1), 20:40 (CF2), 40:20 (CF3), 60:0 (CF4), and 100:100 (CF5).
4 Characterizations
The samples synthesized were grounded and subjected to various characterizations. The structure and the average crystallite size of cobalt ferrite NPs were carried out using X-ray diffractometer (XRD) Model Bruker-axs 104025-0 with Cu-Kα radiation. The powdered samples were scanned over a 2θ range from 10° to 80° at a step size of 0.02°. The morphology of cobalt ferrites with and without capping agent was studied using transmission electron microscopy (TEM) (JEOL/JEM 1200 EX II). The compositional details of the samples were achieved using energy dispersive X-ray spectrometer (EDS) model ZEISS EVO MA10. Fourier transform infrared spectra were recorded using Perkin Elmer spectrophotometer (Spectrum RX1) in the range of 4000–400 cm−1. In addition, the magnetic hysteresis loop of the samples was recorded at room temperature using a vibrating sample magnetometer (5TminiVSM from Cryogenic Ltd.) with the maximum applied field of 7.2 kOe.
5 Results and discussion
5.1 The structural properties of cobalt ferrite NPs
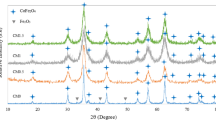
X-ray diffraction patterns of the cobalt ferrite NPs are shown in Fig. 1. The samples synthesized with and without capping agents are closely matched with standard powder diffraction data JCPDS No: 22-1086 and exhibit cubic spinel structure with a space group of Fd-\(\overline {3}\)m (227). The reflections that correspond to (111), (220), (311), (222), (400), (422), (511), (440) and (533) are observed and no other distinct diffraction is found. It confirms that single phase cobalt ferrite NPs are achieved. Also, it is important to notice that the X-ray diffraction does not vary after the addition of PEG, urea and the combination of them, indicates that the capping agents do not change the phase formation of cobalt ferrite NPs as it is reported for oleic acid and fucan coated cobalt ferrite NPs [3]. The broad and well defined structural reflections exhibit the samples are composed of ultrafine particles and/or crystallite size.
The average crystallite size of cobalt ferrite NPs is calculated using Scherrer’s equation from the most intense reflections and the values of average crystallite size, and the lattice constant are listed in Table 1. It is clear that the average crystallite size of cobalt ferrite NPs synthesized without capping agent is 12.1 ± 0.1 nm and the sample CF1 synthesized with urea (60 mg) as capping agent is found to be 14.7 ± 3.5 nm. It is important to notice that the samples synthesized with PEG and the mixture of PEG and urea exhibit almost similar crystallite size with minor variations of about 10.4–11 nm. Similarly, no major deviation is observed in the lattice constant. However, the lattice parameters are comparable to the values reported in the literature [20]. The above results reveal that the selected amount of PEG and the mixture of PEG and urea for the formation of cobalt ferrite NPs do not favor the nucleation and crystal growth which results in the same range of crystallite size. The PEG molecules are easily absorbed on the surface of nanoparticles will greatly decrease the crystal growth rate and confined the crystallite size within 10–11 nm. However, the crystallite size of CF0 and CF1 is somewhat larger than others conveys that the absence of capping agents and/or the addition of only urea promote the crystallite growth of nanoparticles. Therefore, the presence of the capping agents and their concentration in the chemical reaction will modify the growth kinetics of the process, thus control over the size of nanoparticles [21, 22].
5.2 The morphology and elemental composition of cobalt ferrite NPs
The effect of capping agents on the morphology, particle size and its distribution of cobalt ferrite nanoparticles are discussed in the following section. TEM micrographs, selected area energy diffraction (SAED) and particle size distribution (histogram) of cobalt ferrite NPs are shown in Fig. 2. A clear solid ring pattern observed in the SAED confirms the formation of polycrystalline NPs and the d-spacing of the rings is matches with cubic spinel structure of cobalt ferrite NPs. TEM micrographs display similar morphological features in the presence and absence of capping agents. They exhibit nearly spherical particles. Further, it conveys that the capping agents (PEG and urea) and their combined ratio do not change the direction of particle growth thus nearly uniform morphology is attained. The nanoparticles are aggregated to some extent due to the magnetic particle interaction, where the capping agents on the surface of nanoparticles do not show significant prevention of aggregation. However, it shows an appreciable effect on the particle size distribution as shown in the inset of Fig. 2. The ImageJ software has been used to estimate the particle size of nanoparticles where 60–70 particles are considered from the different micrographs of the sample. The population of NPs is normalized from 0 to 1 and Gaussian function is used for the fitting. The mean particle size estimated from the size distribution is found to be 12.0 ± 1.1, 14.3 ± 1.7, 13.4 ± 2.3, 13.4 ± 6.4, 15.3 ± 3.0, and 14.0 ± 2.4 nm for the samples CF0, CF1, CF2, CF3, CF4, and CF5, respectively. The particle size estimated for the samples CF0 and CF1 is similar to the crystallite size calculated from X-ray diffraction. However, the particle size of other samples is slightly higher than the crystallite size. The different histograms shown in Fig. 2 conveys that the capping agents show an effect on the particle size of cobalt ferrite NPs and have narrow size distribution as follows: 5–19, 7–24, 11–25, 8–28, 6–23, and 10–25 nm for CF0, CF1, CF2, CF3, CF4 and CF5, respectively.
Figure 3 shows the EDS spectra of cobalt ferrite NPs and the atomic weight % of the elements Co, Fe, O, C and N are listed in Fig. 3 as an inset. The ratio of Co:Fe is found to be 0.50, 0.51, 0.52, 0.54, 0.53, 0.52, and 0.51 for bulk, CF0, CF1, CF2, CF3, CF4, and CF5, respectively. It shows that the values do not vary significantly with the increase of capping agents and the samples are within the stoichiometric ratio of theoretical value, 1:2 of Co:Fe. In addition, the energy peaks that correspond to carbon and nitrogen are observed approximately at 0.27 and 0.39 keV, respectively confirms that PEG and urea are present in the samples except for CF0. Further, the atomic weight percentage of carbon is more when both PEG and urea are used for the synthesis. Additionally, the presence of the capping agents in cobalt ferrite NPs is supported by functional group analysis in the following section.
5.3 Functional groups analysis and proposed interaction mechanism
The IR-vibrational spectra and possible functional groups of cobalt ferrite NPs are shown in Fig. 4. The sample CF0 synthesized in the absence of capping agents shows vibrations at 3363, 1637, 1518, 1340, 900, 550 and 461 cm−1. The broad and weak absorption band at 3363,1637 and 900 cm−1 correspond to –OH symmetric stretching, H–O–H bending and O–H out of plane bending, respectively of water vapor adsorbed on the nanoparticles from the atmosphere [23, 24]. Weak and less intense vibrations observed at 1518 and 1340 cm−1 are attributed to N–O stretching which may result from the minor traces of nitro compounds [24]. A strong absorption band observed at 550 cm−1 and weak vibration at 461 cm−1 (shown in inset (c)) confirm the metal–oxygen (M–O) Fe3+–O2− or Co2+–O2− stretching vibration of spinel cobalt ferrite at (A) site and [B] site, respectively [6, 13, 25] Cobalt ferrite NPs synthesized with capping agents also exhibit the vibrations observed in the sample CF0. In addition to that, the presence of very low intense absorption band at ~ 2981 cm−1 confirms the asymmetric C–H stretching of PEG [13, 26] in CF2, CF3, CF4 and CF5 and which is not significantly observed in CF0. Also, the vibrations that correspond to C–N asymmetric stretching and NH2 asymmetric bending of urea are evidenced from the weak absorptions at 1474–1494 and 1633–1639 cm−1, respectively [27] in cobalt ferrite samples except for CF0. It is unfortunate that the C–N stretching and NH2 stretching falls within the vibration of N–O stretching and H–O–H bending at almost similar frequency range. And it is difficult to visualize them separately. The above results confirm the presence of PEG and urea on the surface of cobalt ferrite NPs and they show an effect on the intensity and strength of functional group vibrations. With the increase of PEG and decrease of urea concentration, the intensity of ~ 2981 cm−1 that correspond to asymmetric C–H stretching of PEG and C–N stretching of urea at 1474–1526 cm−1 slightly varies and attains maximum for the sample CF5 as shown in Fig. 4a, b. Similarly, with the effect of capping agents, the intensity of M–O vibrations at 543–559 and 452–463 cm−1 varies as shown in Fig. 4c. Also, it is observed that the bandwidth of vibration changes. The bandwidth of the sample CF5 is shifted to a high frequency of 559 and 463 cm−1 for tetrahedral and octahedral sites, respectively. It may due to the possible difference in the bond lengths of M–O vibrations within (A) and [B] sites as reported in the literature [6, 13, 25, 28, 29]. Furthermore, the absorption band of samples is shifted to higher wavenumber, from 543 to 559, 904–915, 1361–1389, 1474–1494, 1633–1639, and 3334–3352 cm−1 confirms the change in the interaction of capping agents on the surface of nanoparticles. A similar observation has been reported for PEG assisted synthesis of zinc ferrite nanoparticles [28].
The formation and interaction mechanism between the cobalt ferrite NPs and capping agents are depicted in Fig. 5. The dropwise addition of aqueous sodium hydroxide in the solution mixture of metal nitrates and capping agents initiate the nucleation process thereby the formation of Co(OH)2 and FeOOH at ambient condition. The precipitated suspension is exposed to the MW irradiation for 7 min. Within a few seconds to 1 min of irradiation, the burst of nucleation attains and reaches saturation due to the uniform distribution of heat within the solution container. The longer duration of MW irradiation promotes the crystal growth and formation of single phase cobalt ferrite (CoFe2O4) NPs. However, the possible interaction between the capping agents and crystallites hinders the crystal growth process and retains almost similar shapes and narrow size distribution as it is evidenced by TEM analysis. Because the strong hydrophilic nature of PEG hydroxyl polymer restrains the migration and adsorption of hydrated cobalt and iron ions on the surface of crystal nucleus [1]. Though, the signatures of PEG and urea on the surface of cobalt ferrite NPs are confirmed using IR spectrum, the less intense vibrations that correspond to capping agents evidence that a very thin layer of PEG and/or urea are adsorbed by electrostatic interaction of –OH groups as shown in Fig. 5. The less intense IR vibrations may due to the removal of hydrophilic PEG and urea during the repeated washing procedure. As it is evidenced by Fig. 4, the presence of physically adsorbed –OH group (1633–1639 cm−1) over the nanoparticles promotes the interaction between the capping agent and cobalt ferrite NPs. However, the interaction between them is weak. Similar interaction mechanism was reported in urea-modified Si nanoparticles [30]. Importantly, as observed in TEM, the increase of capping concentration within the mentioned ratios does not significantly alter the particle size, and shape. Instead, they convert the hydrophobic cobalt ferrite into hydrophilic NPs which will be useful for the magnetic-based biomedical applications.
5.4 Magnetic properties of cobalt ferrite NPs
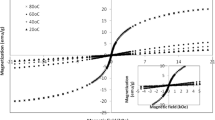
Figure 6 shows the magnetization versus field hysteresis loops of cobalt ferrite NPs at room temperature. All the samples exhibit soft ferromagnetic nature and the different shapes of hysteresis evidence the influence of capping agents on the magnetic properties. As the magnetization of the samples is not saturated with the applied magnetic field, the saturation magnetization (\({M_s}\)) is estimated from the extrapolation of M versus \(1/{H^2}\) at high field region [31] as shown in the inset of Fig. 6. The magnetic properties, the saturation magnetization (\({M_s}\)), remanence magnetization (\({M_r}\)) and coercivity (\({H_c}\)) are extracted from Fig. 6 and correlated with the capping agents and crystallite size as shown in Fig. 7a, b.
The crystallite size variation with and without capping agents discussed in the structural analyses conveys that the kinetics of chemical reaction changes with respect to the nature of capping agents and the electrostatic interaction of hydroxyl groups over the surface of nanoparticles. Figure 7a shows that the average crystallite size and saturation magnetization (\({M_s}\)) follow a similar trend with respect to the different ratio of capping agents, PEG and urea. It reveals that the \({M_s}\) of cobalt ferrite nanoparticles is strongly depending on the crystallite size. In addition, the different ratio of capping agents control the crystal growth rate thereby the crystallite size of cobalt ferrite nanoparticles which directly affect the magnetic properties. The \({M_s}\) of sample CF1, synthesized with urea as a capping agent attains a maximum of 58.3 emu/g compared to others. And the sample synthesized only with PEG shows the magnetization of 55.2 emu/g, which is similar to \({M_s}\) of the sample CF0, synthesized without capping agent. It is found that the magnetization of the samples is less than that of the corresponding bulk CoFe2O4 (80–93 emu/g) [5]. Also, it is less than the value ~ 62 emu/g reported for PEG assisted cobalt ferrite NPs of size 15 ± 3 nm [16]. The results convey that the capping agents, urea, PEG and the mixture of them vary the magnetization of cobalt ferrite NPs from 51.2 to 58.3 emu/g and the magnetization of cobalt ferrite NPs (CF0) synthesized without capping agent falls within this range. Figure 7b shows the average crystallite size dependent magnetic properties of cobalt ferrite NPs. It conveys that the \({M_s}\) of the sample increases with average crystallite size. The higher saturation magnetization of CF1 is due to the larger crystallite size of 14.7 nm compared to others with relatively smaller crystallite size. At larger crystallite size, the magnetic domain size increases thereby the number of magnetic dipole moments aligned along the field direction increases thus results in overall higher magnetization [6]. However, the samples CF2 and CF5 with the crystallite size of 10.9 and 10.5 nm, respectively shows the same value of \({M_s}\) of 53.5 emu/g which is higher than the value 51.2 emu/g of the sample CF3 with the similar crystallite size of 10.7 nm. It is reported that, in addition to the particle size effect, other factors such as the particle–particle interaction, thickness of the dead layer, capping agents over the surface of nanoparticles and divalent (Co2+) and trivalent (Fe3+) cation distribution among the tetrahedral (A) and octahedral [B] sites affect the net magnetization of cobalt ferrite NPs [32]. In the present case, we believe that the capping agents and the possible dead layer formation due to their functional groups over the nanoparticle surface might play a role. The presence of capping agents is supported by FTIR analysis.
Figure 7b conveys that the remanence magnetization (\({M_r}\)) and coercivity (\({H_c}\)) of the samples do not increase with the increase of the crystallite size. However, the (\({M_r}\)) and (\({H_c}\)) follow a similar trend with the crystallite size variation. The sample CF3 synthesized with the mixture of PEG (40 mg) and urea (20 mg) shows higher (\({M_r}\)) and (\({H_c}\)) of 11.3 emu/g and 311.6 Oe, respectively compared to other samples. The low values of remanence and coercivity are due to the presence of magnetization pinning defects incorporated into the nanocrystallites with increase of crystallite size, low the magnetic anisotropy constant, and the distribution of Co2+ ions in [B] site as reported in the literature [32,33,34]. The \({M_r}\) and \({H_c}\) values are varies from 9.8 to 11.63 emu/g and 211.3–311.6 Oe, respectively. The above results convey that the interaction between the capping agents and cobalt ferrite NPs show an effect on\({M_s}\), \({M_r}\) and \({H_c}\) values. Moreover, the addition of only urea improves the magnetization but PEG do not influence on the \({M_s}\) value when compared to the unmodified cobalt ferrite nanoparticles. Further, the suitable selection of urea and PEG ratio and different molecular weight of PEG would be useful to fine tune the magnetic properties at the desired value. Cobalt ferrite NPs synthesized through the present simple route in a short reaction time of 7 min exhibit appreciable magnetization values compared to the samples synthesized at longer duration followed by subsequent calcination processes [35,36,37,38].
6 Conclusions
PEG and urea-modified cobalt ferrite nanoparticles were synthesized through microwave assisted co-precipitation method for the short duration of 7 min. The effect of capping agents on the structural and magnetic properties was discussed. All the samples exhibited single phase cubic spinel structure without any secondary phases. The structural analysis revealed that PEG and the combination of urea and PEG do not significantly promote the nucleation and crystal growth rate of particle formation rather it retained the average crystallite size of cobalt ferrite NPs within 10–11 nm. The hydrophilic nature of the capping agents restrains the movements of the hydroxyl cobalt and iron ions within the solution thus hindered the metal ion coating over CoFe2O4 crystal nucleus thereby slowed down the crystal growth. However, it was found that urea-modified cobalt ferrite NPs exhibited the higher average crystallite size of 14 nm when compared to others. The cobalt ferrite nanoparticles synthesized using the selected ratio of capping agents exhibited almost similar morphological features with nearly narrow size distribution and good stoichiometric ratio. The weak IR vibrations observed at ~ 2981, ~ 1637, and ~ 1474 cm−1 confirmed the presence of PEG and urea on the surface of cobalt ferrite nanoparticles through the possible electrostatic interaction of –OH groups. The intensity variation and shift in the absorption band observed in the IR spectrum supported the possible interaction between the capping agents and nanoparticle surface. Additionally, the change in the intensity of vibration at 543–559 and 452–463 cm−1 revealed that there was a possible variation of the bond length of metal–oxide vibrations at tetrahedral and octahedral sites, respectively. PEG and urea do not alter the nature of magnetism but the magnetic properties of cobalt ferrite nanoparticles were affected by the crystallite size which was controlled by the presence of capping agents. Overall, the higher magnetization and coercivity of 58.3 emu/g and 235.8 Oe, respectively was obtained for the sample synthesized only with urea (60 mg) as a capping agent. Similarly, the appreciable coercivity and magnetization of 311.6 Oe and 51.2 emu/g, respectively was achieved if the mixture of PEG (40 mg) and urea (20 mg) were used. The hydrophilic nature with significant magnetic properties of cobalt ferrites realized in the present simple and short reaction route would be a better choice in biomedical, smart ferrofluids and other magnetic applications.
References
M. Sun, A. Zhu, Q. Zhang, Q. Liu, J. Magn. Magn. Mater. 369, 49 (2014)
J. Yang, P. Zou, L. Yang, J. Cao, Y. Sun, D. Han, S. Yang, Z. Wang, G. Chen, B. Wang, X. Kong, Appl. Surf. Sci. 303, 425 (2014)
P.L. Andrade, V.A.J. Silva, J.C. Maciel, M.M. Santillan, N.O. Moreno, L. De Los Santos Valladares, A. Bustamante, S.M.B. Pereira, M.P.C. Silva, J. Albino Aguiar, Hyperfine Interact. 224, 217 (2014)
T. Prabhakaran, R.V. Mangalaraja, J.C. Denardin, J. Magn. Magn. Mater. 444, 297 (2017)
S. Ammar, A. Helfen, N. Jouini, F. Fievet, I. Rosenman, F. Villain, P. Molinie, M. Danot, J. Mater. Chem. 11, 186 (2001)
T. Prabhakaran, R.V. Mangalaraja, J.C. Denardin, J.A. Jiménez, Ceram. Int. 43, 5599 (2017)
F.J. Pedrosa, J. Rial, K.M. Golasinski, M. Rodr, RSC Adv. 6, 87282 (2016)
I. Bilecka, M. Niederberger, Nanoscale 2, 1358 (2010)
S. Komarneni, M.C. D’Arrigo, C. Leonelli, G.C. Pellacani, H. Katsuki, J. Am. Ceram. Soc. 81, 3041 (2005)
J.A. Gerbec, D. Magana, A. Washington, G.F. Strouse, J. Am. Chem. Soc. 127, 15791 (2005)
T. Prabhakaran, R.V. Mangalaraja, J.C. Denardin, Mater. Res. Express 5, 026102 (2018)
S. Li, G.W. Qin, T.W. Pei, Y. Ren, Y. Zhang, C. Esling, L. Zuo, J. Am. Ceram. Soc. 92, 631 (2009)
A.S. Nikolić, N. Jović, J. Rogan, A. Kremenović, M. Ristić, A. Meden, B. Antić, Ceram. Int. 39, 6681 (2013)
A. Abbasi, H. Khojasteh, M. Hamadanian, J. Mater. Sci. Mater. Electron. 27, 4972 (2016)
S. Jovanović, M. Spreitzer, M. Tramšek, Z. Trontelj, D. Suvorov, J. Phys. Chem. C 118, 13844 (2014)
S. Esir, R. Topkaya, A. Baykal, Ö Akman, M.S. Toprak, J. Inorg. Organomet. Polym. Mater. 24, 424 (2014)
R. Talebi, J. Mater. Sci. Mater. Electron. 28, 9749 (2017)
K. Hedayati, S. Azarakhsh, D. Ghanbari, J. Nanostruct. 6, 127 (2016)
T. Yu, Z. Wu, W.-S. Kim, RSC Adv. 4, 37516 (2014)
T. Prabhakaran, R.V. Mangalaraja, J.C. Denardin, J.A. Jiménez, J. Alloys Compd. 716, 171 (2017)
M.R. Parra, F.Z. Haque, Optik 126, 1562 (2015)
M. Sudha, S. Senthilkumar, R. Hariharan, A. Suganthi, M. Rajarajan, J. Sol-Gel. Sci. Technol. 65, 301 (2013)
M. Mozaffari, S. Manouchehri, M.H. Yousefi, J. Amighian, J. Magn. Magn. Mater. 322, 383 (2010)
N. Wu, L. Fu, M. Su, M. Aslam, K.C. Wong, V.P. Dravid, Nano Lett. 4, 383 (2004)
M. Chithra, C.N. Anumol, B. Sahu, S.C. Sahoo, J. Magn. Magn. Mater. 424, 174 (2017)
C. Zhang, M.R. Salick, T.M. Cordie, T. Ellingham, Y. Dan, L.-S. Turng, Mater. Sci. Eng. C 49, 463 (2015)
R. Keuleers, H.O. Desseyn, B. Rousseau, C. Van Alsenoy, J. Phys. Chem. A 103, 4621 (1999)
F. Gözüak, Y. Köseoǧlu, A. Baykal, H. Kavas, J. Magn. Magn. Mater. 321, 2170 (2009)
S.M. Ansari, R.D. Bhor, K.R. Pai, S. Mazumder, D. Sen, Y.D. Kolekar, C.V. Ramana, ACS Biomater. Sci. Eng. 2, 2139 (2016)
B.N. Hao, Y.X. Guo, Y.D. Liu, L.-M. Wang, H.J. Choi, J. Mater. Chem. C 4, 7875 (2016)
G. Bertotti, in Hysteresis in Magnetism, ed. by G. Bertotti (Academic Press, San Diego, 1998), pp. 297–346
E.P. Wohlfarth, J. Appl. Phys. 29, 595 (1958)
T. Prabhakaran, J. Hemalatha, Ceram. Int. 42, (2016)
Y. Köseoğlu, A. Baykal, F. Gözüak, H. Kavas, Polyhedron 28, 2887 (2009)
J. Peng, M. Hojamberdiev, Y. Xu, B. Cao, J. Wang, H. Wu, J. Magn. Magn. Mater. 323, 133 (2011)
M. Houshiar, F. Zebhi, Z.J. Razi, A. Alidoust, Z. Askari, J. Magn. Magn. Mater. 371, 43 (2014)
Y.M. Abbas, S.A. Mansour, M.H. Ibrahim, S.E. Ali, J. Magn. Magn. Mater. 323, 2748 (2011)
L. Cui, P. Guo, G. Zhang, Q. Li, R. Wang, M. Zhou, L. Ran, X.S. Zhao, Colloids Surf. A 423, 170 (2013)
Acknowledgements
This work was supported by FONDECYT Postdoctoral Research Project No.: 3160170, FONDECYT Project No.: 1140195, and CONICYT BASAL CEDENNA FB0807, Government of Chile.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Prabhakaran, T., Mangalaraja, R.V., Denardin, J.C. et al. The effect of capping agents on the structural and magnetic properties of cobalt ferrite nanoparticles. J Mater Sci: Mater Electron 29, 11774–11782 (2018). https://doi.org/10.1007/s10854-018-9276-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9276-9