Abstract
In this work, we have focused the effect of substrate on electrochemically grown copper oxide and copper sulfide thin films. The prepared films have been subjected to X-ray diffraction, scanning electron microscopy, Energy dispersive X-ray analysis, UV–Visible spectroscopic techniques for the determination crystalline nature, morphology, composition and optical properties. X-ray diffraction results indicated the deposited films exhibited cubic structure with most reflection along (110), (220) planes for copper oxide and copper sulfide. Scanning electron microscopy along with energy dispersive analysis by X-rays showed that films with uniform morphology and nearly stoichiometry have been obtained for film obtained on SnO2 substrate. Optical absorption and transmittance measurements showed that the deposited films exhibited band gap value of 2.28 and 2.45 eV for copper oxide and copper sulfide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For the past few years, there has been growing interest in metal oxides and metallic alloys for its wide range of applications in opto electronic devices and solar cells [1,2,3,4,5,6,7,8,9,10]. Among the former ones, cuprous oxide (Cu2O) is a p-type semiconductor attracted many researchers due to the reason that it has direct band gap semiconductor with an energy gap value in the range between 2.0 and 2.35 eV [7]. Copper sulfide with stoichiometric phases Cu2S and CuS attracted many researchers due to its applications in solar absorber coatings, photo thermal conversion and solar energy conversion devices. Cuprous sulphide (Cu2S) is a p-type semiconductor with a ideal band gap value in the range between 1.2 and 2.5 eV which make them interesting as absorber material in solar cells due to its low cost with non toxic nature [9, 10]. Selman et al. have prepared Cu2O thin films on glass and Si substrates by frequency magnetic reactive sputtering method and investigated its structural, morphological and optical properties, respectively [2]. Dolai et al. [3] have prepared Cu2O thin films by d.c reactive sputtering technique and investigated its properties using X-ray diffraction, scanning electron microscopy, X-ray photoelectron spectroscopy and Raman spectroscopic techniques, respectively. Cu2O thin films prepared by microwave-activated reactive sputtering technique and its properties were analyzed using X-ray diffraction, X-ray photoelectron spectroscopy, UV–Visible and Raman spectroscopic techniques and reported by Alajlani et al. [4]. Liu et al. have prepared Cu2O thin films by pulsed laser deposition method and determined its properties by X-ray diffraction, Transmittance and Hall effect measurement techniques, respectively [5]. Cu2O thin films prepared using thermal oxidation of copper layer and its absorption coefficient, band gap and photoluminescence properties were determined by Karapetyan et al. [6]. Badillo-Ávila were determined structural, morphological, and optical properties of Cu2O thin films annealed at different temperatures in the range between 300 and 550 °C. They have reported that the prepared films possess band gap value in the range between 1.20 and 2.35 eV [7]. Ozaslan et al. prepared Cu2O thin films using SILAR method and investigated its properties by XRD, XANES and UV–Visible spectroscopic techniques, respectively [8].Cu2S thin films prepared on glass substrates by chemical bath deposition technique and its UV–Visible and current voltage characteristics are reported by Patil et al. [9]. Sabah have reported the effect of annealing on structural, morphological and electrical properties of Cu2S thin films deposited on different substrates [10]. When compared to the techniques mentioned electrochemical deposition has been evidenced as a powerful ability to control the crystallization of Cu2O, Cu2S and it presents a simple, economical, cost effective, less time consuming as well as the stoichiometry of the deposited films should be easily controlled by adjusting the deposition parameters as well as composition of the electrolytic bath etc., [11,12,13].
In the present investigation, Cu2O and Cu2S thin films are prepared on stainless steel (SS), aluminium (Al) and tin oxide (SnO2) substrates using an electrodeposition technique. Electrochemical behaviour of the films has been analyzed using cyclic voltammetry. Structural, morphological properties of the prepared films are characterized using X-ray diffraction and scanning electron microscopy. Energy dispersive X-ray analysis has been carried out to determine composition of the deposited films. Optical absorption and transmittance measurements are determined using an UV–Visible spectroscopic analysis to find out the parameters such as band gap, refractive index, extinction coefficient of the deposited films. The effect of substrate on structural, morphological, compositional and optical properties of the deposited films are investigated.
2 Experimental details
The chemicals used in the present work were of AR grade reagents. Thin films of Cu2O and Cu2S were prepared by electrochemical reduction process of 0.02 M CuSO4, 0.1 M tartaric acid and 0.1 M CuSO4, 0.005 M Na2S2O3 for Cu2O and Cu2S with three electrode cell configuration consists of SS, Al, SnO2 substrates, platinum electrode and saturated calomel electrode (SCE) as working, counter and reference electrode, respectively. Initially, the pH value of the electrolytic bath was found to be 4.5 ± 0.1. The pH value of the electrolytic bath was adjusted to 9.0 ± 0.1 and 2.5 ± 0.1 by the addition of flexible amount of KOH and H2SO4 for the deposition of Cu2O and Cu2S. The substrates were cleaned ultrasonically in a bath of isopropanol, acetone followed by distilled water for 15 min. The SCE was kept into the solution by luggin capillary arrangement whose tip was closed as possible to the working electrode. The deposition potential was found to be in the range between 0 and − 400 mV versus SCE, 0 and − 550 mV versus SCE for Cu2O and Cu2S. The deposition time was found to be in the range between 0 and 60 min. The temperature of the electrolytic bath was found to be in the range between 50 and 80 °C for all depositions.
Growth mechanism of the deposited films was analyzed by an in-situ characterization cyclic voltammerty employing Potentiostat/Galvanostat (SP 50 Bio Logic, France) with SS, Al, and SnO2 substrates. Thickness of the deposited films was estimated by Stylus profilometry (Mitutoyo SJ 301, Japan). Structural properties was determined from X-ray diffraction data which is obtained from X-ray diffractometer (XPERT PRO PANalytical, Netherland) with CuKα radiation (λ = 0.15418 nm). Film composition with surface morphology was analyzed using an Energy dispersive X-ray analysis set up attached with scanning electron microscope (JEOL JSM 840). An optical absorbance and transmittance was analyzed using UV–Visible-NIR (Schimadzu, Japan) spectrometer to find out the associated optical properties of the deposited films.
3 Results and discussion
3.1 Cyclic voltammetry
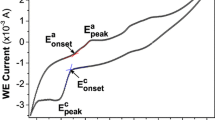
Growth of thin films using electrochemical deposition is based on the formation of solid phase from an electrolytic bath involves nucleation and growth processes. The process of heterogeneous reaction takes place thus leads to produce films with certain thickness value due to the combination of particles on the surface of the substrate. Cyclic voltammetric curves recorded for Cu2O thin films prepared on SS and SnO2 substrates which is obtained from an alkaline bath consists of 0.02 M CuSO4 with 0.1 M tartaric acid is shown in Fig. 1. It is noted that the appearance of oxidation peak at + 300 mV versus SCE indicated the reduction of metallic Cu2+ ion to metal Cu. The pH value was increased to 9.5 ± 0.1 by the addition of an adjustable amount of KOH to the electrolytic bath. The observation of reduction peak shifted towards negative region and the appearance of cathodic reduction peak at − 350 mV versus SCE, results the formation of Cu2O on the substrate according to Eq’s from (1) to (3) mentioned below.
Initially, CuSO4·5H2O decomposed into CuSO4 according to Eq. (1). Thereafter, decomposed CuSO4 reacts with tartaric acid in the electrolytic bath leads to produce the complex of CuSO4 (Tartaric). The concentration of OH− ion in the electrolytic bath adjusted the pH value to 9.5 ± 0.1, show the way of formation of Cu2O due to the redox reaction of Cu2+ with OH. The appearance of anodic peak at + 330 mV versus SCE may be due to the oxidation of Cu2O from Cu(OH)2 and CuO. As a result, the pH value of the electrolytic bath determines the formation of metal oxides by the technique of electrodeposition. It is observed from Fig. 2, that the appearance of cathodic reduction peak at − 350 mV versus SCE indicated the reaction of Cu metal with O thus leads to produce CuO films on the substrate of SS and SnO2. It is evident from Figure that the formation of Cu takes place in the potential range between + 300 and − 550 mV versus SCE.
The mechanism of formation of Cu2S thin films on Al and SnO2 substrate is described by the following Eq’s (4)–(9). The formation of Cu2S is more complex process, since they involved several thermodynamic problems such as poor adhesion of film to the substrate due to the process of hydrogen evolution whereas electrolytic bath with more acidic nature produces peel off film from the substrate. In this process, the deposition of less noble constituent of the alloy takes place first, in which its reduction potential is quite closer to the standard reduction potential of S2O32− according to Eq. (7). The formation of Cu2S thin films takes place according to Eq’s from (5) to (10).
where \(E{}_{{{\text{Cu}}}}\) and \(E{}_{{\text{S}}}\) are the potential of the working electrode in a given solution, \(E_{{{\text{Cu}}}}^{0}\), \(E_{{\text{S}}}^{0}\) are the standard electrode potential for Cu and S with respect to SCE, R is an universal gas constant, T is absolute temperature of the electrolytic bath, N is the number of electrons required for the reduction and F is Faraday constant. The deposition of Cu occurs at potentials more positive than their standard reduction potential by gaining free energy in the compound formation. The formation of Cu2S thin film takes place with negative free energy \(\Delta G= - 86.19\;{\text{kJmo}}{{\text{l}}^{ - 1}}\). Cyclic voltammogram of Cu2S thin films on Al and SnO2 substrates those are obtained from an electrolytic bath consisting of 0.05 M CuSO4 and 0.05 M Na2S2O3. The voltammetric curves recorded with in the potential range between + 1000 and − 1000 mV versus SCE with a scan rate of 20 mV/s is shown in Fig. 2. The observation of oxidation peak at + 540 mV versus SCE which may be due to the oxidation of Cu2+ to metal Cu according to Eq. (5) It is also observed that the reduction peak I at − 325 mV versus SCE which may be due to the formation of Cu2S on the substrate. It is noted that the reduction peak at − 40 mV versus SCE which may be due to the process of reduction of solvent in the electrolytic bath. The observation of slight shift in reduction potential which may be due to the usage of different substrates for deposition.
3.2 Stylus profilometry
Thickness of the deposited films has been controlled by adjusting the deposition parameters such as potential, time of deposition, plating current, bath temperature and pH value of the electrolytic bath [11]. The temperature of the electrolytic bath is found to be in the range between 50 and 80 °C for even deposition of film on the substrate of SS, Al and SnO2. Variation of films thicknes with deposition time for Cu2O thin films obtained on SS and SnO2 substrate is shown in Fig. 3. It is noted that film thickness increases linearly with deposition time and attained its maximum value at a deposition time of 40 min. If the deposition time is increased above 40 min, the rate of deposition is found to decrease thus results decrease in value of film thickness which is indicated in Fig. 3. Similar behaviour is exhibited for Cu2S thin films obtained on Al and SnO2 substrates which is shown in Fig. 4. The higher value of film thickness is obtained at a time of deposition of 40 min for both Cu2O, Cu2S, respectively.
3.3 Structural analysis
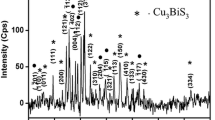
Structural properties of Cu2O and Cu2S thin films have been determined using X-ray diffractometer with the diffraction angle in the range between 20 and 80. XRD pattern showed that the prepared films possess polycrystalline nature with cubic structure for Cu2O and Cu2S. XRD pattern obtained for Cu2O thin films prepared on SS and SnO2 substrate is shown in Fig. 5. The value of lattice spacing “d” is calculated from the observed 2θ values and compared with standard JCPDS ICDD file for cubic Cu2O [14]. The diffraction peaks of Cu2O are found at 2θ values of angles 37.03, 43.02, 52.39, 62.22, 66,45 corresponding to the lattice planes (111), (200), (211), (220), (221) respectively. It is observed from Fig. 5a,b that the height of the peak observed at 2θ value 37.13, 52.39 is found to be higher than all other peaks in the diffractogram indicated that the crystallites are preferentially oriented along (111) and (211) plane for films obtained on SnO2 and SS substrates. XRD pattern recorded for Cu2S thin films on Al and SnO2 substrate is shown in Fig. 6. The value of interplanar spacing “d” is calculated from the observed 2θ values and compared with standard JCPDS ICDD file for cubic Cu2S [15]. The height of (111) plane is found to be higher than all other peaks in the diffraction pattern indicated that the crystallites have most prominent reflection along (111) plane. The observation of considerable increase in peak height of preferential plane (111) represents the usage of different substrates for deposition. Crystallite size is defined as the sizes of the crystallites formed along the surface of the substrate [11, 12]. Debye Scherrer formula is used to calculate the value of crystallite size from the FWHM data which is obtained from XRD pattern [13]. Strain is defined as the restoring force which can be acts on the surface of the substrate to restrict the formation of crystallites on its surface [12, 13]. Dislocation density is defined as the number of dislocation lines per unit volume of the crystal [12, 13]. The estimated value of crystallite size and dislocation density for Cu2O and Cu2S thin films on different substrates are given in Table 1.
3.4 Surface morphology and composition
Surface morphology of Cu2O thin films on SnO2 substrate is shown in Fig. 7. It is observed that the surface is covered with small uniform spherical shaped grains. It is observed that the sizes of the grains are found to be in the range between 0.7 and 0.9 µm. SEM image of Cu2S thin films obtained on SnO2 substrate is shown in Fig. 8. It is noted that the substrate covered with unshaped crystal grains as shown in Fig. 8. The observation of slight change in surface morphology which may be due to usage of different substrate for deposition. The sizes of the grains are found to be in the range between 500 and 700 nm. This value of atomic percentage in EDX results indicated, the that the results is consistent with X-ray diffraction analysis of the sample with phase corresponds to Cu2O and Cu2S. There is slight shift in value of atomic percentage is noted for films prepared on different substrates (Table 1).
3.5 Optical absorption analysis
Optical absorption analysis have been carried out by absorption and transmittance measurements for Cu2O and Cu2S thin films prepared on SnO2 substrates using Schimadzu UV–Visible-NIR spectrophotometer within the wavelength range between 300 and 1100 nm. The value of absorption coefficient for the deposited films which has been calculated using Eq. (10) [13]
where α is the absorption coefficient in cm−1, t is thickness of the deposited film in nm, A and T are the percentage of absorbance and transmittance of the deposited films. The nature of transition present in the deposited film is calculated using Eq. (11) [12, 13].
where (hν) is photon energy in eV, Eg is an band gap energy in eV, A is an energy dependent constant and n is an integer. The percentage of transmittance for Cu2O and Cu2S thin films obtained on SnO2 in shown in Figs. 9 and 10. A plot of (hν) versus (αhν)2 for Cu2O and Cu2S films prepared on SnO2 substrate is indicated inset of Figs. 9, 10. It is observed that linear portion of the plot is extrapolated to X (energy)-axis gives band gap value of the deposited films. The band gap value is found to be 2.28 and 2.45 eV for the deposited films of Cu2O and Cu2S.
4 Conclusions
Cu2O and Cu2S thin films were successfully prepared on SS, Al and SnO2 substrates by potentiostatic electrodeposition technique. Structural investigation showed that the deposited layer exhibited cubic structure with preferential orientation along (111) and (200) plane. The observation of lower value of strain and dislocation density indicated that the films deposited on SnO2 substrate exhibited better microstructural properties. Surface morphology showed that the surface covered with uniform spherical shaped grains for films obtained on SnO2 The value of band gap was found to be 2.28 and 2.45 eV for Cu2O and Cu2S. Hence, we have concluded that the films prepared on SnO2 substrate is found to exhibit well oriented microstructural, compositional and optical properties.
References
Kirk-Othemer, Encyclopedia of Chemical Technology, Vol. 20, (Wiley, New York, 1982)
A.M. Selman, M.A. Mahdi, Z. Hassan, Physica E 94, 32 (2017)
S. Dolai, S. Das, S. Hussain, R. Bhar, A.K. Pal, Vacuum 141, 296 (2017)
Y. Alajlani, F. Placido, A. Barlow, H.O. Chu, S. Song, S.U. Rahman, R.D. Bold, D. Gibson, Vacuum 144, 217 (2017)
X. Liu, M. Xu, X. Zhang, W. Wang, X. Feng, A. Song, Appl. Surf. Sci. 435, 305 (2018)
A. Karapetyan, A. Reymers, S. Giorgio, C. Fauquet, L. Sajti, S. Nitsche, M. Nersesyan, V. Gevorgyan, W. Marine, J. Lumin. 159, 325 (2015)
M.A. Badillo-Ávila, R. Castanedo-Pérez, G. Torres-Delgado, J. Márquez-Marín, O. Zelaya-Ángel, Mater. Sci. Semicond. Process. 74, 203 (2018)
D. Ozaslan, O.M. Ozkendir, M. Gunes, Y. Ufuktepe, C. Gumus, Optik 157, 1325 (2018)
M. Patil, D. Sharma, A. Dive, S. Mahajan, R. Sharma, Proc. Manuf. 20, 505 (2018)
F.A. Sabah, N.M. Ahmed, Z. Hassan, H.S. Rasheed, Proc. Chem. 19, 15 (2016)
S. Thanikaikarasan (in press) Ionics. (https://doi.org/10.1007/s11581-017-2292-6)
P. Jeyakumar, S. Thanikaikarasan, B. Natarajan, J. Mater. Sci. Mater. Electron. 28, 2538 (2017)
S. Thanikaikarasanan, T. Mahalingam, M. Raja, S. Velumani, Mater. Sci. Semicond. Proc. 37, 215 (2015)
Joined Council for Powder Diffracted System International Centre for Diffraction Data 2003, Pennsylvania. PDF No. 77-0199
Joined Council for Powder Diffracted System International Centre for Diffraction Data 2003, PDF No. 20-1225, Pennsylvania
Acknowledgements
The corresponding author (Dr. S. Thanikaikarasan) gratefully acknowledge the financial support received from the Board of Research in Nuclear Sciences, Department of Atomic Energy (BRNS-DAE), Mumbai, India with File No. 2012/34/13/BRNS/No.166 for carrying out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thanikaikarasan, S., Perumal, R., Sankaranarayanan, K. et al. Electrochemical, microstructural, compositional and optical characterization of copper oxide and copper sulfide thin films. J Mater Sci: Mater Electron 29, 15529–15534 (2018). https://doi.org/10.1007/s10854-018-9109-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9109-x