Abstract
We report the facile synthesis of zinc oxide (ZnO) nanostructures with different sizes and morphologies by a rapid microwave assisted synthesis using ethylenediaminetetraacetic acid (EDTA) and/or trisodium citrate as chelating agents and their characterization. The obtained ZnO nanostructures having hexagonal Wurtzite structure with different morphologies. With the aid of EDTA and/or trisodium citrate, flowers, flakes, solid spheres and porous spheres were obtained by controlling the crystal growth habit and the concentration of ZnO growth units under microwave irradiation. The optical behaviour was analyzed using UV–Vis spectroscopic technique which indicates that the prepared ZnO nanostructures exhibit band gap between 3.27 and 3.37 eV due to potential fluctuations in electronic band structure of ZnO owing to surface-related defects and/or adsorbed species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zinc oxide (ZnO) is one of attractive II–VI compound semiconductor material due to its wide direct band gap (3.37 eV) and large excitonic binding energy (60 meV) at room temperature [1, 2]. Generally, ZnO crystallizes in the hexagonal Wurtzite-type structure at ambient conditions, in which the Zn or O atoms are tetrahedrally coordinated to 4-O (or Zn) atoms. Owing to its unique chemical and physical characteristics, ZnO is widely used in various applications such as gas sensors, light emitting diodes, field effect transistors, ultraviolet lasers, photodetectors, solar cells, photo catalysts, and so on [1,2,3,4,5,6,7]. The physical and chemical characteristics of ZnO nanostructures are significantly influenced by size and morphology and they play an important role on the above mentioned applications [1,2,3,4,5,6,7]. Hence, lot of processing techniques such as sol–gel, biosynthesis, hydrothermal, co-precipitation, electrochemical deposition, microwave assisted method, etc., have been developed to prepare ZnO nanostructures with different sizes and morphologies [7,8,9,10,11,12,13]. It is important to mention that surfactants/chelating agents such as methenamine, polyvinyl alcohol (PVA), trisodium citrate, ethylenediaminetetraacetic acid (EDTA), ethylenediamine, cetyltrimethyl ammonium bromide (CTAB) and polyethylene glycol (PEG) were widely employed to control the size and shape of ZnO nanostructures during the precipitation in above methods.
Trisodium citrate is a typical anionic chelating agent and it can be used to obtain different ZnO micro/nanostructures. Citrate anions can easily chelate with divalent zinc ions, thereby controlling the growth of ZnO during the precipitation [13,14,15]. Similarly, EDTA is a strong complex reagent and it can form stable complex with zinc ions which effectively controlled the growth of ZnO micro/nanostructures during the period of precipitation [8, 16].
Microwave is a part of electromagnetic radiation in the frequency range 0.3–300 GHz. The commonly used frequency and energy of microwave in laboratory oven for synthesis is 2.45 GHz and 10−5 eV, respectively [17, 18]. Under microwave heating, microwave photonic energy is transferred to the reaction mixture via the interaction of the electromagnetic field at the molecular level resulting in rapid volumetric heating [17, 18]. Consequently synthesis of controlled nanostructures using microwave technique has received much attention from researchers among the various synthesis methods. This method offer some unique characteristics such as rapid volumetric heating, high reproducibility, high purity, high yield, control in size and shape of nanostructures [17, 18]. The present study is focused on rapid and facile synthesis of different ZnO nanostructures using trisodium citrate and/or EDTA under microwave irradiation and their optical properties towards the optoelectronic applications.
2 Experimental procedure
Chemicals such as zinc nitrate [Zn(NO3)2], trisodium citrate (C6H5Na3O7·2H2O), sodium hydroxide (NaOH), EDTA were obtained from Merck, India and used without further purification. Distilled water was used as the solvent. Zinc oxide nanostructures with different morphologies were prepared by microwave assisted method as follows: 0.1 M Zn(NO3)2 was first dissolved in 100 ml distilled water under constant stirring and then 100 ml of chelating agent with 0.01 M concentration was added with zinc nitrate solution. Consequently, 0.5 M NaOH was added to adjust the pH of reaction mixture to 10. The obtained reaction mixture was then put into microwave oven and irradiated at 800 W for 15 min. After microwave irradiation, the obtained reaction mixture was centrifuged to remove byproducts and dried at 100 °C for 5 h to get final product. In order to prepare ZnO with different morphology, 0.01 M citrate, 0.01 M EDTA and 0.01 M citrate/EDTA mixture as a chelating agent were added with zinc nitrate before addition of NaOH and obtained samples were named as TZO, EZO and TEZO, respectively. Also ZnO without addition of chelating agent was prepared for comparison and referred as CZO.
The X-ray diffraction (XRD) pattern of samples was obtained using Rigaku MiniFlex II powder X-ray diffractometer with voltage and current setting of 30 kV and 15 mA, respectively. The XRD patterns were recorded in the range 20° ≤ 2θ ≤ 80° using CuKα radiation (1.5406 Å). The FTIR spectrum of prepared samples was obtained using Bruker Tensor 27 FT-IR spectrometer in the range of 400–4000 cm−1 with the aid of KBr technique. JSM JEOL-6390 scanning electron microscope (SEM) and JSM JEOL-2100 transmission electron microscope (TEM) were used to examine the morphological features of the prepared samples. The elementals present in the prepared sample were analyzed using Oxford INCA energy dispersive X-ray fluorescence (EDX) micro analyzer. The optical absorption by the prepared samples is measured using UV–Vis–NIR Spectrophotometer (Make: Varian, Model: Cary 5000).
3 Results and discussion
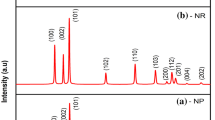
Crystalline phases present in the prepared samples was determined by comparing the experimental XRD pattern with Join Committee Powder Diffraction standard (JCPDS) data compiled by International Centre for Diffraction Data (ICDD). XRD pattern of synthesized samples are shown in Fig. 1. The observed 2θ values of diffraction peaks for all the samples well matched with standard JCPDS data for ZnO (JCPDS No. 89-1397). All XRD patterns shows the presence of diffraction peaks around 2θ values of 31.6°, 34.3°, 36.1°, 47.4°, 56.5°, 62.9°, 66.3°, 67.8°, 69.1°, 72.4° and 76.9° corresponds to (002), (100), (101), (102), (110), (103), (200), (112), (201), (004) and (202) Miller’s planes of ZnO, respectively. This observation indicates that the prepared samples contain only ZnO as crystalline phase having hexagonal Wurtzite structure. However significant variation in the intensity of diffraction peaks (Fig. 1) were observed between samples prepared with different chelating agents which indicates that chelating agents plays an important role in the growth of ZnO crystallites. The lattice parameters (a and c) of the ZnO crystalline phase having hexagonal Wurtzite structure were calculated using the following relation [19].
where d is the spacing between the planes in the atomic lattice. The interplanar spacing (d) was calculated according to Bragg’s equation (2d sinθ = nλ). The volume (V) of the hexagonal unit cell was determined by using the following relation V = 0.866 a2c [19]. The calculated lattice parameters and unit cell volume are shown in Table 1. The value of a decreased and c increased when trisodium citrate or EDTA or combination of trisodium citrate/EDTA were added during the synthesis of ZnO. Moreover, addition of chelating agents has significantly reduced the volume of unit cell and increased the lattice distortion (c/a). These results indicate that chelating agents significantly control the crystallization of ZnO by formulating an interaction with free zinc ions during the microwave synthesis.
FTIR spectrum of the sample acquired at 400–4000 cm−1 is shown in Fig. 2. The band between 400 and 555 cm−1 correspond to stretching vibrations of Zn–O bond which is typical characteristic band of ZnO with Wurtzite hexagonal structure [6, 19, 20]. The broad band extending from 2600 to 3700 cm−1 and a strong band at 1620 cm−1 are attributed to the stretching and bending modes of the adsorbed water molecules by the prepared samples, respectively. The peaks located at 2844 and 2918 cm− 1 are due to the symmetric and asymmetric C–H bonds, respectively. The stretching vibration of C–O is observed at 884 cm−1. The peak at 1375 cm−1 is attributed to symmetric vibrations C–O bond. The band at 1450 cm−1 is overlap of both C–H and C–O bonds. The band at 2350 cm−1 is due to absorption of atmospheric CO2 molecules by prepared samples [6, 19, 20]. Hence water molecules along with some organic impurity may be adsorbed on prepared zinc oxide nanostructures during the synthesis process under microwave irradiation. According to Akhavan et al. the surface of the ZnO synthesized by chemical route is usually covered by OH groups resulting in formation of a thin layer of Zn(OH)2 [21]. FTIR results of all samples clearly shows that presence of vibration due to OH of adsorbed water molecules indicating the surface of the ZnO is covered by a thin layer of Zn(OH)2.
Zinc oxide nanostructures with different size and morphologies can be produced by microwave assisted precipitation method with aid of various chelating agents. Morphological features of prepared ZnO nanostructures at different magnifications are shown in Fig. 3. It is found that CZO consist of microflowers (Fig. 3a, b) in which leaf-like petals having 100–300 nm width and 0.5–1 µm length are extending radially from center. These leaf-like petals are composed of very fine nanocrystal which is evident from its TEM image (Fig. 3c). Figure 3d, e obviously reveal that EZO consist of irregular shaped nanoflakes with dimension of about 100–300 nm. TEM image shown in Fig. 3f indicates that these irregular nanoflakes are composed of nanocrystals with irregular shape. Conversely, TZO is composed of solid microsphere with large number of spikes projecting outward from a core in all directions which is clear from its SEM images (Fig. 3g, h). TEM image shown in Fig. 3i evidently indicates that the spikes are made of fine nanocrystals. Figure 3j clearly demonstrates that TEZO contains uniform porous microspheres with diameter of about 0.5-1 µm. Magnified SEM image in Fig. 3k shows that this microspheres are constructed from nanosheets having 10–20 nm thickness. TEM image in Fig. 3i illustrates the morphological feature of individual nanosheet.
Figure 4 shows the EDX spectrum of prepared ZnO nanostructures which clearly indicate that Zn and O are present in prepared samples. The calculated elemental composition is shown in Table 2. The EDX spectrum revealed that atomic ratio of Zn to O in all samples were about 1 which was consistent with stoichiometric ratio of ZnO and it effectively proves the formation of stoichiometric zinc oxide.
UV–Vis absorbance spectra of the prepared ZnO nanostructures in the wavelength range of 200–1200 nm are shown in Fig. 5. It is important to note that significant variation was observed between the samples prepared with different chelating agents. The optical band gap (Eg) of the semiconductor is related with the incident photon energy and optical absorption coefficient (α) as follows [19, 22, 23].
where A is a proportionality constant and n is a constant exponent which is equal to ½ for ZnO semiconducting materials. As a consequence, the following relation is obtained
Using the above relation, graphs were plotted between (αhν)2 and hν and the linear region was extrapolated to (αhν)2 = 0 to obtain a value of the energy gap. A plot between (αhν)2 versus hν for the prepared ZnO nanostructures by microwave irradiation with aid of chelating agents is shown in Fig. 6 and the values of the band gap obtained for CZO, TZO, EZO and TEZO are 3.29 ± 0.05, 3.32 ± 0.04, 3.27 ± 0.03 and 3.37 ± 0.05 eV, respectively. It is found that band gap energy is significantly influenced by method of processing as well as size and morphology of ZnO nanostructures. This may be due potential fluctuations in electronic band structure of ZnO due to surface-related defects and/or adsorbed species [22, 23].
There are several reports available on synthesis of ZnO nanostructures using various additives/modifiers [6, 13, 23,24,25,26,27,28,29]. Table 3 shows the recent progress in synthesis of ZnO nanostructures by microwave irradiation method using various additives/modifiers. To the best of our knowledge, there is no report on the effect of EDTA and/or trisodium citrate under microwave treatment. However, the effect of EDTA and trisodium citrate on the formation of ZnO nanostructure under hydrothermal treatment was well established [8, 13]. In this study, we have studied the effect of EDTA and/or trisodium citrate on the formation of different ZnO nanostructures under microwave treatment for the first time. Microwave heating is an alternative method for conventional heating which induce rapid nucleation leads to formation of complex ZnO nano/microstructures. In other words, the role of microwave is not only to accelerate the reaction between the precursor materials but also to lead to formation of three dimensional complex ZnO nano/microstructures. When sodium hydroxide was added with zinc nitrate solution, the zinc cations can react with hydroxide anions and form stable Zn(OH)42− complexes which could act as the growing unit for growth of ZnO nanostructures [9, 10, 30]. Hence, formation of ZnO from zinc nitrate and sodium hydroxide mixture under microwave irradiation is as follows.
The formation of zinc oxide nanostructure mainly depends upon the number of active sites that are available on formed Zn(OH)42− species and the number of growth species surrounding those sites [9, 10, 30]. Under microwave irradiation, growth of the ZnO is in a random way which leads to formation of flower-like 3D structure as shown in Fig. 3b.
Citrate anions have one to three carboxylate groups in solution depending on the pH of the medium. They can easily generate complexes with Fe3+, Ca2+, Ag+, Mg2+, and Zn2+ ions [13,14,15]. Thus, citrates have been used as organic modifier in the synthesis of nanocrystals with controlled size and morphologies [31]. When trisodium was introduced in zinc nitrate solution, the zinc cations can form zinc citrate and control the formation of Zn(OH)42− species and growth of ZnO units. Consequently solid microsphere with large number of spikes was formed as shown in Fig. 3.
EDTA, a member of the polyamino carboxylic acid family, is a complex reagent and it forms metal–EDTA complexes with metal precursors [8, 16]. When EDTA was added with zinc nitrate, the zinc cations can strongly chelate with EDTA and form stable Zn-EDTA complexes at base condition which inhibit the development of Zn(OH)42− species and formation of ZnO. Under microwave heating, the microwave energy about 10−5 eV is transferred to Zn/EDTA/NaOH mixture at molecular level by the interaction of the electromagnetic field resulted in rapid volumetric heating. Due to rapid microwave heating, Zn ions were released from Zn-EDTA complexes rapidly and develop Zn(OH)42− species under base condition in rapid manner. Consequently irregular shaped ZnO nanoflakes were formed due to heterogeneous nucleation and growth of ZnO units under rapid microwave heating. On the other hand, porous microspheres constructed from nanosheets were obtained when EDTA and trisodium citrate were added with zinc nitrate solution during microwave synthesis. The chelating effect of EDTA and/or trisodium citrate plays a key role in controlling the size and morphology of ZnO nanostructures. The size and morphology controlled ZnO nanostructures were obtained in this study by controlling the crystal growth and the concentration of ZnO growth units with the aid of EDTA and/or trisodium citrate under microwave irradiation.
4 Conclusion
A rapid and facile microwave assisted method was developed to synthesize ZnO nanostructures with different sizes and morphologies. With the aid of EDTA and/or trisodium citrate, flowers, flakes, solid spheres and porous spheres were obtained by controlling the crystal growth habit and the concentration of ZnO growth units under microwave irradiation. Moreover, the obtained ZnO nanostructures exhibit band gap between 3.27 and 3.37 eV due potential fluctuations in electronic band structure of ZnO owing to surface-related defects and/or adsorbed species. Consequently, developed method can be a potential method to obtain ZnO nanostructures for wide spectrum of applications.
References
C. Klingshirn, ZnO: material, physics and applications. Chem. Phys. Chem. 8, 782–803 (2007)
A. Kołodziejczak-Radzimska, T. Jesionowski, Zinc oxide–from synthesis to application: a review. Materials 7, 2833–2881 (2014)
C.W. Litton, D.C. Reynolds, T.C. Collins, Zinc Oxide Materials for Electronic and optoelectronic device applications (Wiley, New York, 2011)
E. Guziewicz, K. Kopalko, G. Łuka, M.I. Łukasiewicz, T. Krajewski, B.S. Witkowski, S. Gierałtowska, Zinc oxide for electronic, photovoltaic and optoelectronic applications. Low Temp. Phys. 37, 235 (2011)
A. Pimentel, J. Rodrigues, P. Duarte, D. Nunes, F.M. Costa, T. Monteiro, R. Martins, E. Fortunato, Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: a photocatalytic study. J. Mater. Sci. 50, 5777–5787 (2015)
T. Krishnakumar, R. Jayaprakash, N. Pinna, N. Donato, A. Bonavita, G. Micali, G. Neri, CO gas sensing of ZnO nanostructures synthesized by an assisted microwave wet chemical route. Sens. Actuators B 143, 198–204 (2009)
Z. Petrović, M. Ristić, S. Musić, The effect of sodium polyanethol sulfonate on the precipitation of zinc oxide. J. Alloys Compd. 694, 1331–1337 (2017)
Z. Li, Y. Fang, L. Peng, D. Pan, M. Wu, EDTA-assisted synthesis of rose-like ZnO architectures. Cryst. Res. Technol. 45, 1083–1086 (2010)
C. Wang, Y. Gao, L. Wang, P. Li, Morphology regulation, structural, and photocatalytic properties of ZnO hierarchical microstructures synthesized by a simple hydrothermal method. Phys. Status Solidi A 214, 1600876 (2017)
Y.A. Sumanth, R.A. Sujatha, S. Mahalakshmi, P.C. Karthika, S. Nithiyanantham, S. Saravanan, M. Azagiri, Synthesis and characterization of nanophase zinc oxide materials. J. Mater. Sci.: Mater. Electron. 27, 1616–1621 (2016)
J. Huang, C. Xia, L. Cao, X. Zeng, Facile microwave hydrothermal synthesis of zinc oxide one-dimensional nanostructure with three-dimensional morphology. Mater. Sci. Eng. B 150, 187–193 (2008)
F. Li, L. Hu, Z. Li, X. Huang, Influence of temperature on the morphology and luminescence of ZnO micro and nanostructures prepared by CTAB-assisted hydrothermal method. J. Alloys Compd. 465, 14–19 (2008)
S. Cho, S. Jung, K. Lee, Morphology controlled growth of ZnO nanostructures using microwave irradiation: from basic to complex structures. J. Phys. Chem. C 112, 12769–12776 (2008)
S. Cho, J. Jang, S. Jung, B.R. Lee, E. Oh, K. Lee, Precursor effects of citric acid and citrates on ZnO crystal formation. Langmuir 25, 3825–3831 (2009)
M. Venkatesh, G.S. Kumar, S. Viji, S. Karthi, E.K. Girija, Microwave assisted combustion synthesis and characterization of nickel ferrite nanoplatelets. Mod. Electron. Mater. 2, 74–78 (2016)
G.S. Kumar, J. Akbar, R. Govindan, E.K. Girija, M. Kanagaraj, A novel rhombohedron-like nickel ferrite nanostructure: microwave combustion synthesis, structural characterization and magnetic properties. J. Sci.: Adv. Mater. Dev. 1, 282–285 (2016)
I. Bilecka, M. Niederberger, Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2, 1358–1374 (2010)
Y. Zhu, F. Chen, Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 114, 6462–6555 (2014)
T. Prakash, G. Neri, A. Bonavita, E.R. Kumar, K. Gnanamoorthi, Structural, morphological and optical properties of Bi-doped ZnO nanoparticles synthesized by a microwave irradiation method. J. Mater. Sci.: Mater. Electron. 26, 4913–4921 (2015)
X. Zhao, L. Qi, Rapid microwave-assisted synthesis of hierarchical ZnO hollow spheres and their application in Cr(VI) removal. Nanotech. 23, 235604 (2012)
O. Akhavan, M. Mehrabian, K. Mirabbaszadeh, R. Azimirad, Hydrothermal synthesis of ZnO nanorod arrays for photocatalytic inactivation of bacteria. J. Phys. D: Appl. Phys. 42, 225305 (2009)
Y. Köseoğlua, A simple microwave-assisted combustion synthesis and structural, optical and magnetic characterization of ZnO nanoplatelets. Ceram. Inter. 40, 4673–4679 (2014)
A. Manikandan, E. Manikandan, B. Meenatchi, S. Vadivel, S.K. Jaganathan, R. Ladchumananandasivam, M. Henini, M. Maaza, J.S. Aananda, Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: synthesis structural optical and antibacterial studies. J. Alloys Compd. 723, 1155–1161 (2017)
L. Tong, Y. Liu, H. Rong, L. Gong, Microwave-assisted synthesis of hierarchical ZnO nanostructures. Mater. Lett. 112, 5–7 (2013)
W. Wang, Y. Zhu, Shape-controlled synthesis of zinc oxide by microwave heating using an imidazolium salt. Inorg. Chem. Commun. 7, 1003–1005 (2004)
M. Ma, Y. Zhu, G. Cheng, Y. Huang, Microwave synthesis and characterization of ZnO with various morphologies. Mater. Lett. 62, 507–510 (2008)
Y. Cao, B. Liu, R. Huang, Z. Xia, S. Ge, Flash synthesis of flower-like ZnO nanostructures by microwave-induced combustion process. Mater. Lett. 65, 160–163 (2011)
N.F. Hamedani, A.R. Mahjoub, A.A. Khodadadi, Y. Mortazavi, Microwave assisted fast synthesis of various ZnO morphologies for selective detection of CO, CH4 and ethanol. Sens. Actuators B 156, 737–742 (2011)
M. Debbarma, S. Das, M. Saha, Effect of reducing agents on the structure of zinc oxide under microwave irradiation. Adv. Manufact. 1, 183–186 (2013)
M.L.D. Peralta, J.G. Serrano, U. Pal, Morphology defined ZnO nanostructures through microwave assisted chemical synthesis: growth mechanism, defect structure, and emission behaviours. Adv. Sci. Lett. 6, 159–166 (2012)
S. Das, K. Dutta, A. Pramanik, Morphology control of ZnO with citrate: a time and concentration dependent mechanistic insight. Cryst. Eng. Comm. 15, 6349–6358 (2013)
Acknowledgements
G. Suresh Kumar would like to express his sincere thanks to University Grant Commission, India for financial support through minor research project scheme [File No: 4-4/2015-16 (MRP/UGC SERO)]. The authors express their special thanks to STIC, Cochin, India for providing TEM and UV-DRS facilities for characterizing the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duraimurugan, J., Kumar, G.S., Venkatesh, M. et al. Morphology and size controlled synthesis of zinc oxide nanostructures and their optical properties. J Mater Sci: Mater Electron 29, 9339–9346 (2018). https://doi.org/10.1007/s10854-018-8964-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-8964-9