Abstract
Dy3+-doped and Dy3+/Eu3+ co-doped NaBi(MoO4)2 phosphors were successfully synthesized using a high temperature solid-state method. The crystal structure and phase purity of these as-prepared samples were characterized by X-ray diffraction. Under 453 nm excitation, NaBi(MoO4)2:Dy3+ phosphors exhibited the characteristic weak blue emission peak at 488 nm and strong yellow emission peak at 577 nm, which originate from the 4F9/2 → 6H15/2 and 4F9/2 → 6H13/2 transitions of Dy3+ ions, respectively. Therefore, a white light could be composed of a blue light from chip and yellow emission of NaBi(MoO4)2:Dy3+ phosphor. Additionally, color-tunable emissions were observed in the spectra of NaBi(MoO4)2:Dy3+, Eu3+ phosphors due to efficient energy transfer from Dy3+ to Eu3+ ions. By varying co-doping concentrations of Dy3+ and Eu3+ ions, the emission color of NaBi(MoO4)2:Dy3+, Eu3+ could be varied for high quality blue-excited white light-emitting diodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
White light-emitting diodes (LEDs) are considered as a new generation of light source owing to their high efficiency, long operational lifetime and low environmental impact [1,2,3,4,5]. Most commercially available white LEDs are achieved by combining a blue-emitting LED chip with yellow phosphor (Y3Al5O12:Ce3+) [2]. However, this type of white LEDs has a poor color rendering index (CRI) and high correlated color temperature (CCT) due to the color deficiency in red region [6,7,8,9]. Furthermore, the most common commercial red-emitting phosphors, such as Y2O2S:Eu3+, are inefficient and chemically unstable [6]. Thus, the white LEDs with blue/green/red tricolor phosphors excited by near-ultraviolet LED chips are regarded as an alternative light source, and have some excellent advantages like good color uniformity, high CRI and excellent light quality [10, 11]. However, these white LEDs also have problems including the strong absorption of blue light by red and green phosphors, low luminous efficiency and unstable color spots [12,13,14]. To overcome these limitations, a suitable single-phase white-emitting phosphor is needed for application in white LEDs.
Over the past few decades, trivalent rare earth ions doped molybdate compounds have been extensively studied in the fields of phosphors, photocatalysts and optical temperature sensors due to their excellent luminescence properties and chemical stability [15,16,17,18,19,20,21,22]. Among these molybdates, NaBi(MoO4)2 crystal with scheelite structure is considered suitable phosphor host due to their excellent physical and chemical properties [23]. The most common method of single-phase white light generation is co-doping different activators into the same crystal matrix, such as Dy3+–Eu3+ and Eu2+–Mn2+ [24, 25]. Dy3+ and Eu3+ can emit blue, yellow and red light with energy transfer due to 4F9/2 → 6H15/2, 4F9/2 → 6H13/2 and 5D0 → 7F2 transitions, respectively, white light has been achieved in single-phase phosphors such as LiLa(MoO4)2:Dy3+, Eu3+ [26], ZANP glass:Dy3+, Eu3+ [27] and CsGd(WO4)2:Dy3+, Eu3+ [28]. However, the photoluminescence and concentration quenching of NaBi(MoO4)2:Dy3+ phosphors have not been systematically reported, and the investigation of luminescence properties of single-phase NaBi(MoO4)2:Dy3+, Eu3+ white phosphor is significant interest for development of high quality white LEDs.
In this paper, a series of NaBi(MoO4)2:Dy3+ phosphors was prepared using conventional solid state reaction. Effects of the synthesis temperature and Dy3+ concentration on the luminescence properties of Dy3+-doped NaBi(MoO4)2 phosphor were studied. Furthermore, Eu3+ ions were co-doped in NaBi(MoO4)2:Dy3+ to obtain color-tunable emission in yellow–red region. Finally, photoluminescence spectra and the energy transfer mechanisms between Dy3+ and Eu3+ ions, and Dy3+ and Bi3+ ions, were investigated.
2 Experimental
All NaBi(MoO4)2:Dy3+, Eu3+ samples were prepared via the traditional solid-state reaction method. (NH4)6Mo7O24·4H2O was used as the molybdenum source, Bi2O3 as the bismuth source, Eu2O3 as the europium source, Dy2O3 as the dysprosium source and Na2CO3 as the sodium source. All the starting materials were used without further purification. Firstly, all starting materials were weighed according to stoichiometry and grinded thoroughly in an agate mortar to mix them together. Then, the mixture was transferred to a crucible and heated at a constant temperature within the range of 650–850 °C for 3 h in a furnace. At last, all as-prepared phosphors were cooled naturally to room temperature and grinded again.
X-ray diffraction patterns of the prepared phosphors were measured using an X-ray diffractometer (DX-2700, Aolong Company, China) with Cu-Kα radiation (λ = 1.5406 Å). The photoluminescence (excitation and emission) spectra and lifetimes of samples were performed via using a luminescence Hitachi F-4600 spectrophotometer with a 150 W xenon lamp as the excitation source. Scanning electron microscope (SEM) and Energy disperse spectroscopy (EDS) (ProX, Phenom, Netherlands) were used to describe the morphology and composition of as-prepared samples. All measurements were performed at room temperature and the air environment.
3 Results and discussion
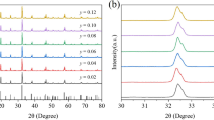
Figure 1a shows the XRD patterns of NaBi(MoO4)2:0.01Dy3+ phosphors synthesized at different temperatures (650–850 °C). The diffraction peaks of all prepared samples are matched perfectly with the standard diffraction peaks of NaBi(MoO4)2 phase (PDF#51-1508), which has a scheelite structure. The grain size could be calculated by the Debye–Scherrer formula [29]:
Here, D is the average thickness of grain perpendicular to the crystal plane, K is the Scherrer constant (K = 0.89), λ is the X-ray wavelength of the Cu Kα radiation (λ = 1.54 Å), B is the full-width-at-half-maximum of the diffraction peaks (112) and θ is the corresponding diffraction angle (θ = 28.45°). According to the equation, the average grain diameters at 650, 700, 750, 800 and 850 °C were calculated to be 10.19, 10.75, 11.21, 11.63 and 11.90 Å, respectively. It indicated that the average crystal size of NaBi(MoO4)2:0.01Dy3+ increased with the increased synthesis temperature because more thermal energy would contribute to the formation of more NaBi(MoO4)2 molecule on NaBi(MoO4)2 crystal.
To further explore the structure of as-prepared NaBi(MoO4)2:Dy3+ phosphor, the Rietveld refinement of NaBi(MoO4)2:Dy3+ based on the XRD patterns with lattice constants and atom positions were carried by the GSAS program. The corresponding results are presented in Fig. 1b. For the main diffraction peaks there was good agreement between observed and calculated patterns. The refinement results illustrated that crystallized NaBi(MoO4)2:Dy3+ had a tetragonal crystal structure with I41/a space group; its crystal lattice parameters were determined to be a = 5.2738 Å, b = 5.2738 Å, c = 11.5607 Å. The refinement factors converged to Rwp = 21.53%, Rp = 14.54% and χ2 = 11.68, indicating good fitting quality.
The emission spectra of NaBi(MoO4)2:0.01Dy3+ phosphors prepared at different temperatures were obtained (Fig. 2) to determine the influence of synthesis temperature on the luminescence properties of phosphor. It can be seen that the synthesis temperature had no effect on the emission peak type or position, but cause the emission intensity varied greatly. With the increase of synthesis temperature from 650 to 750 °C, the luminescence intensity increased. However, when the synthesis temperature was further increased to 800 °C, the luminescence intensity tended to decrease. The synthesis temperature had two opposite effects on the structure and luminescence properties of these samples. On the one hand, the increase of synthesis temperature is beneficial to the doping of Dy3+ ions into crystal lattice and promotes the crystallization of NaBi(MoO4)2 with a more complete, less defective lattice, which resulting in improved luminescence of the NaBi(MoO4)2:Dy3+ sample. On the other hand, with the increase of synthesis temperature, the agglomeration of crystal increased and many too large grains were formed, which led to decreased luminescence [30, 31]. The former effect was dominant when the synthesis temperature was lower than 750 °C, however, when the synthesis temperature exceeded 750 °C, the latter become a dominant effect. The luminescence intensity depends on the influence magnitude of both effects.
The excitation spectrum (λem = 577 nm) and emission spectrum (λem = 453 nm) of NaBi(MoO4)2:0.05Dy3+ phosphors are depicted in Fig. 3. The excitation spectrum consists of four characteristic peaks located at 389, 426, 453 and 472 nm, corresponding to the 6H15/2 → 4I13/2, 6H15/2 → 4G11/2, 6H15/2 → 4I15/2 and 6H15/2 → 4F9/2 transitions of Dy3+ ions, respectively. The strong excitation peak at 453 nm demonstrated that the NaBi(MoO4)2:0.05Dy3+ phosphors were readily excited by blue light. Under 453 nm excitation, the sample exhibited a strong yellow emission at 577 nm and a weak peak at 488 nm due to the 4F9/2 → 6H13/2 and 4F9/2 → 6H15/2 transitions of Dy3+ ions [26, 32]. In general, yellow emission would be strong and blue emission become weak when Dy3+ ions occupy non-inversion center, while the strong and weak relation would be transformed if Dy3+ ions occupied highly symmetric positions. As a result, the stronger yellow emission at 577 nm indicated that Dy3+ ions occupied the lowly symmetry position with non-inversion in the NaBi(MoO4)2 lattice [32]. There was an abnormal phenomenon that characteristic excitation peaks of Dy3+ located at 353 nm and 366 nm were extremely weak (seen from inset of Fig. 3) and the peak at 387 nm become lower in intensity comparing to usual excitation of Dy3+. These results could be explained by the special energy absorption of Bi3+: when bismuth compounds are irradiated by light with a higher energy than the band gap, a separation of conduction band electrons and valence band holes is generated (see Fig. 4) [33]. As a result, the energy in the excitation level is absorbed by the Bi3+ ions, and the probability of the excited electrons of Dy3+ returning to the ground state is greatly reduced, which result in the weak emission and excitation. Bi3+–O2− in NaBi(MoO4)2 has a band gap of 3.1 eV [34, 35] and the Bi3+electrons can be excited to the conduction band when the energy is greater than 3.1 eV (˂ 398 nm), which means the abnormal reduction of excitation intensity would happened in the peaks lower than 398 nm.
Figure 5 depicts the emission spectra (λex = 453 nm) of NaBi(MoO4)2:Dy3+ phosphors containing different Dy3+ concentrations. As shown, emission intensities increased gradually with increased Dy3+ ion concentration, the maximum emission intensity was appeared at 5 mol% Dy3+ doping concentration. When the concentration exceeded 5 mol%, the emission intensity decreased due to concentration quenching via the cross-relaxation (CR) mechanism. The increase of Dy3+ concentration reduces the distance between adjacent Dy3+ ions, which makes the probability of energy transfer gradually increase. As far as the CR mechanism is concerned, there are several possible channels [36]:
Firstly, the electron of Dy3+ ion in a highly excited state (6H15/2) is unstable and emits energy, which would be absorbed by the adjacent Dy3+ in the ground state (4F9/2). Then, the electrons at the 4F9/2 level are excited via these three cross-relaxation processes to a metastable level. Eventually, all electrons back to the ground state and there is no photon emitted in the whole process.
In order to better understand the influence of the distance between adjacent Dy3+ ions on the concentration quenching of luminescence, the critical Dy3+ distance that can identified the type of the interaction mechanism was calculated by Eq. (2). It’s known from Blasse’ report, the critical distance can be expressed as [37]:
Here, RC is the critical distance, V is the volume of the unit cell, xc is the critical concentration of Dy3+ ions and Z is the number of cations. There are two different mechanisms: multipole–multipole interaction and exchange interaction. When the critical distance is larger than 5 Å, the multipole–multipole interaction is dominant; conversely, it would become to the exchange interaction when the RC smaller than 5 Å. For NaBi(MoO4)2:Dy3+ sample, V = 321.36 Å3, xc = 0.05 and Z = 2, RC was calculated to be about 19.3 Å. Therefore, the dominant energy transfer (ET) mechanism of the NaBi(MoO4)2:Dy3+ phosphors was multipole–multipole interaction.
According to the theory of Dexter for multipolar interaction and Reisfeld’s approximation, the following relation can be given [28, 38]:
where c is scalar constant, I is the luminescence intensity of NaBi(MoO4)2:xDy3+ (x = 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07), and Q = 3, 6, 8 or 10, corresponding to nearest-neighbor ions, dipole–dipole (D–D), dipole-quadrupole (D–Q) and quadrupole–quadrupole (Q–Q) interactions, respectively. It can be seen in Fig. 6 that the dependence of log(I/x) on log(x) is linear and the slope is − 0.72. Thus, Q was calculated to be 2.16, which was closest to 3, the main concentration quenching mechanism of NaBi(MoO4)2:xDy3+ phosphors was determined to be via nearest-neighbor ions interaction.
The micromorphology and elemental composition of the as-prepared NaBi(MoO4)2:0.05Dy3+, 0.05Eu3+ phosphor sample obtained by SEM and EDS is shown in Fig. 7. It is observed that the particles have smooth morphology and the diameters are ranging from 7 to 10 µm. The elemental composition of NaBi(MoO4)2:0.05Dy3+, 0.05Eu3+ sample verified by EDS shows (at.%) Na 7.39%, Bi 8.72%, Dy 0.53%, Eu 0.37%, Mo 14.92% and O 68.07%. The stoichiometric atom ratio of each element in NaBi(MoO4)2:0.05Dy3+, 0.05Eu3+ is Na 8.26%, Bi 8.26%, Dy 0.41%, Eu 0.41%, Mo 16.53% and O 66.12%. The composition suggested by EDS is consistent with stoichiometric weight ratio with reasonable relative error.
Figure 8 shows the photoluminescence spectra of NaBi(MoO4)2:0.05Dy3+, 0.05Eu3+ phosphors. The excitation spectrum (λem = 577 nm for Dy3+) of NaBi(MoO4)2:0.05Dy3+, 0.05Eu3+ was similar to that of NaBi(MoO4)2:0.05Dy3+. The excitation spectrum (λem = 617 nm for Eu3+) of NaBi(MoO4)2:0.05Dy3+, 0.05Eu3+ exhibited a strong peak at 464 nm and two weak peaks at 394 and 416 nm, which ascribed to the 7F0 → 5L6, 7F0 → 5D3 and 7F0 → 5D2 transitions of Eu3+ ion, respectively. In addition, the red emission of Eu3+ at 617 nm emission could be generated under the excitation of Dy3+ at 453 nm, which is different to the traditional electron transition of Eu3+, and indicate that the energy was transferred from Dy3+ to Eu3+ ions in NaBi(MoO4)2:0.05Dy3+, yEu3+. The emission spectrum of NaBi(MoO4)2:Dy3+, Eu3+ showed the emission peaks at 487, 577 and 617 nm, which ascribed to the 4F9/2 → 6H15/2 and 4F9/2 → 6H13/2 transitions of Dy3+ ions and 5D0 → 7F2 transition of Eu3+ ions, respectively. These results indicated that the emission of red, yellow and blue light was achieved under blue excitation.
To further research the energy transfer (ET) mechanism in photoluminescence process of Dy3+/Eu3+ co-doped NaBi(MoO4)2, the emission spectra of NaBi(MoO4)2:0.05Dy3+, yEu3+ excited at 453 nm with different Eu3+ co-doping concentrations were illustrated in Fig. 9. There are three characteristic emission peaks of Dy3+ and Eu3+ at 487, 577, and 617 nm, respectively. The corresponding intensity of emission peak varies with the concentrations of Eu3+ co-doping. From the inset of Fig. 9, it can be observed that the intensity of emission peak at 617 nm exhibited an opposite trend compared with excitation at 577 nm. The variation of the emission intensity of NaBi(MoO4)2:xDy3+, yEu3+ demonstrated that energy was transferred from Dy3+ to Eu3+ ions.
Figure 10 shows the schematic energy-level diagram with the ET mechanism of Dy3+ and Eu3+. Under 453 nm excitation, the electron of Dy3+ at 6H15/2 level was excited to the 4I15/2 level, and then jumped to the 4F9/2 level with non-radiative relaxation (NR) which was more stable than 4I15/2. Next, the electron of Dy3+ at 4F9/2 level transfer to the 6H15/2, 6H13/2 and 6H11/2 levels accompanied by characteristic emission at 487, 577 and 665 nm, respectively. Meanwhile, through the resonance between the 4F9/2 and 5D0 levels, part of energy of Dy3+ is transferred to the electron of Eu3+ in 5D0 level. Finally, most of the electrons at 5D0 level returned to the 7F2 level and release red emission at 617 nm.
The luminescence decay curves of NaBi(MoO4)2:0.05Dy3+, yEu3+ phosphors (λex = 453 nm and λem = 617 nm) were measured at room temperature to further identify the ET between Dy3+ and Eu3+, as shown in Fig. 11. The decay curves of NaBi(MoO4)2:0.05Dy3+, yEu3+ phosphors can be well fitted by a double-exponential function given in the equation [39]:
where I(t) corresponds to the luminescence intensity at time t and I0 represents the initial intensity, A1 and A2 are the fitting parameters, both τ1 and τ2 are components of the decay time as a long lifetime and a short lifetime, respectively. The double exponential behavior indicates the heterogeneous distribution of Eu3+ ions in the NaBi(MoO4)2:0.05Dy3+, yEu3+ phosphors. The average lifetime can be evaluated by the next formula [40]:
From Eq. 5 and Fig. 11, the effective lifetime values for NaBi(MoO4)2:0.05Dy3+, yEu3+ phosphors with y = 0.01, 0.03 and 0.05 were calculated as 0.380 ms, 0.408 ms and 0.412 ms, respectively. It is worth noting that the lifetime of luminescence is prolonged with Eu3+ ion concentration increasing, which powerfully prove that there is a significant energy transfer from Dy3+ to Eu3+ ions in NaBi(MoO4)2.
The Commission International de l′Eclairage (CIE) chromaticity coordinates diagram for the luminescence of NaBi(MoO4)2:0.05Dy3+, yEu3+ is shown in Fig. 12. The coordinates of NaBi(MoO4)2:0.05Dy3+, yEu3+ (y = 0, 0.01, 0.02, 0.03, 0.04 and 0.05) are very close to the yellow area because of the strong yellow peak, and located at (0.3982, 0.4339), (0.3996, 0.4290), (0.4035, 0.4271), (0.4049, 0.4243), (0.4053, 0.4208) and (0.4104, 0.4231). As the Eu3+ concentration was increased, the coordinates of NaBi(MoO4)2:Dy3+, Eu3+ gradually tend to the red region. Furthermore, the related CCT values of samples could be calculated by the following expression [41]:
Here, n = (x − xe)/(y − ye), (x, y) is the coordinates of samle, (xe, ye) is the coordinate of epicenter and its value is xe = 0.3320 and ye = 0.1858. From Table 1, it can be seen that the CCT value of NaBi(MoO4)2:0.05Dy3+, 0.05Eu3+ is 3625 K with a good warm white performance while the NaBi(MoO4)2:0.05Dy3+ emits a neutral white light with high CCT value. As results, the CCT value varied from 3932 to 3625 K with the incorporation of Eu3+ ions for compensation of the red light composition, and the CCT value of NaBi(MoO4)2:0.05Dy3+, yEu3+ decreases and its warm white property is improved. Color controllability can be obtained by modulating the ratio of Dy3+ and Eu3+ ion in NaBi(MoO4)2. Therefore, Dy3+/Eu3+ co-doped NaBi(MoO4)2 phosphors have potential applications in color displays and warm white LEDs fields.
4 Conclusions
In summary, a series of Dy3+/Eu3+ co-doped NaBi(MoO4)2 phosphors were successfully synthesized by a solid state reaction method. The excellent structure and luminescence intensity of NaBi(MoO4)2:Dy3+ were obtained under conditions at 750 °C for 3 h. Under 453 nm excitation, the characteristic emissions peaks of Dy3+ and Eu3+ ions were observed in the luminescence spectra of Dy3+/Eu3+ co-doped NaBi(MoO4)2 phosphors. The NaBi(MoO4)2:Dy3+ phosphors get its optimal luminescence intensity when the Dy3+ ion concentration was 5 mol% and the concentration quenching would happen with Dy3+ ion concentration further increasing. Moreover, the emission color of Dy3+/Eu3+ co-doped NaBi(MoO4)2 phosphors could be changed in the yellow-white region by adjusting the radio of Eu3+ ion concentrations. Therefore, the color controlled NaBi(MoO4)2:Dy3+, Eu3+ phosphors are expected to have potential applications in the optical fields such as light display and warm white LEDs devices.
References
P.F. Smet, K. Korthout, J.E.V. Haecke, D. Poelman, Using rare earth doped thiosilicate phosphors in white light emitting LEDs: towards low colour temperature and high colour rendering. Mater. Sci. Eng. B 146, 264–268 (2008)
Q. Yang, G. Li, Y. Wei, H. Chai, Synthesis and photoluminescence properties of red-emitting NaLaMgWO6:Sm3+, Eu3+ phosphors for white LED applications. J. Lumin. 199, 323–330 (2018)
Y. Zhou, Z.J. Zhang, Y.Q. Chu, Y.F. Pan, M.J. You, T.T. Zheng, J.Y. Xu, Phase transition and photoluminescence properties of Eu3+-doped ZnMoO4 red phosphors. Sci. China Technol. Sc. 60, 1473–1479 (2017)
Y.Q. Li, J.E.J.V. Steen, J.W.H.V. Krevel, G. Botty, A.C.A. Delsing, F.J. DiSalvo, G.D. With, H.T. Hintzen, Luminescence properties of red-emitting M2Si5N8:Eu2+(M = Ca, Sr, Ba) LED conversion phosphors. J. Alloys Compd. 417, 273–279 (2006)
A.K. Vishwakarma, K. Jha, M. Jayasimhadri, A.S. Rao, K. Jang, B. Sivaiah, D. Haranath, Red light emitting BaNb2O6:Eu3+ phosphor for solid state lighting applications. J. Alloys Compd. 622, 97–101 (2015)
Q. Zeng, P. He, M. Pang, H. Liang, M. Gong, Q. Su, Sr9R2–xEuxW4O24(R = Gd and Y) red phosphor for near-UV and blue InGaN-based white LEDs. Solid State Commun. 149, 880–883 (2009)
H. Zhu, M. Fang, Z. Huang, Y. Liu, K. Chen, X. Min, Y. Mao, M. Wang, Photoluminescence properties of Li2Mg(WO4)3:Eu3+ red phosphor with high color purity for white LEDs applications. J. Lumin. 172, 180–184 (2016)
H.S. Jang, W.B. Im, C.L. Dong, D.Y. Jeon, S.K. Shi, Enhancement of red spectral emission intensity of Y3Al5O12:Ce3+ phosphor via Pr co-doping and Tb substitution for the application to white LEDs. J. Lumin. 126, 371–377 (2007)
R. Zhang, B. Wang, P. Zhou, X. Wu, X. Huang, B. Wang, A novel Ce3+:Y3Al5O12 and Eu2+:Sr2Si5N8 dual phosphors-in-glass thick film for warm white LED. Mater. Lett. 221, 31–34 (2018)
G. Guo, X. Ding, Y. Xu, Luminescent properties of Eu3+-doped BaLn2ZnO5 (Ln = La, Gd, and Y) Phosphors by the Sol–Gel Method. J. Am. Ceram. Soc. 93, 1708–1713 (2010)
K. Li, M. Xu, J. Fan, M. Shang, H. Lian, J. Lin, Tunable green to yellowish-orange phosphor Na3LuSi2O7:Eu2+, Mn2+ via energy transfer for UV-LEDs. J. Mater. Chem. C 3, 11618–11628 (2015)
R.R.G. Seeta, E. Pavitra, J.S. Yu, Cross-relaxation induced tunable emissions from the Tm3+/Er3+/Eu3+ ions activated BaGd2O4 nanoneedles. Dalton Trans. 43, 9766–9776 (2014)
Y. Tian, N. Feng, M. Wierzbicka-Wieczorek, P. Huang, L. Wang, Q. Shi, C. Cui, Energy transfer-induced tunable emission color and thermal quenching of Ca3Y(PO4)3:Eu2+, Mn2+ phosphor for NUV-pumped white LEDs. Dyes Pigment. 131, 91–99 (2016)
J. Ding, Q. Wu, Y. Li, Q. Long, C. Wang, Y. Wang, Sr7.3Ca2.7(PO4)6F2:Eu2+, Mn2+: a novel single-phased white light-emitting phosphor for NUV-LEDs. Dalton Trans. 44, 9630–9636 (2015)
S. Sinha, K. Kumar, Studies on up/down-conversion emission of Yb3+ sensitized Er3+ doped MLa2(MoO4)4 (M = Ba, Sr and Ca) phosphors for thermometry and optical heating. Opt. Mater. 75, 770–780 (2018)
W. Bi, Q. Meng, W. Sun, S. Lu, Optical temperature sensing properties of Er3+, Yb3+ co-doped NaGd(MoO4)2 phosphor. Ceram. Int. 43, 1460–1465 (2017)
Y. Shi, B. Liu, C. Li, W. Luo, Z. Wang, Effect of W6+ dopant on the morphology and luminescence properties of NaLa(MoO4)2:Eu3+ phosphors. Mater. Res. Bull. 101, 319–323 (2018)
J. Huang, J. Huang, Y. Lin, X. Gong, Y. Chen, Z. Luo, Y. Huang, Spectroscopic properties of Dy3+-doped NaGd(MoO4)2 crystal. J. Alloys Compd. 664, 266–271 (2016)
X. Yan, X. Wang, W. Gu, M.M. Wu, Y. Yan, B. Hu, G. Che, D. Han, J. Yang, W. Fan, W. Shi, Single-crystalline AgIn(MoO4)2, nanosheets grafted Ag/AgBr composites with enhanced plasmonic photocatalytic activity for degradation of tetracycline under visible light. Appl. Catal. 164, 297–304 (2015)
Z. Wang, P. Li, Q. Guo, Z. Yang, Solid-state synthesis and luminescent properties of yellow-emitting phosphor NaY(MoO4)2:Dy3+ for white light-emitting diodes. J. Lumin. 30, 842–846 (2015)
S.-S. Liu, D.-P. Yang, D.-K. Ma, S. Wang, T.-D. Tang, S.-M. Huang, Single-crystal NaY(MoO4)2 thin plates with dominant {001} facets for efficient photocatalytic degradation of dyes under visible light irradiation. Chem. Commun. 47, 8013–8015 (2011)
J. Liu, R. Wei, J. Hu, L. Li, J. Li, Novel Bi2O3/NaBi(MoO4)2 heterojunction with enhanced photocatalytic activity under visible light irradiation. J. Alloys Compd. 580, 475–480 (2013)
N.V. Gusakovaa, A.V. Mudryi, M.P. Demesh, A.S. Yasukevich, A.A. Pavlyuk, A.A. Kornienko, E.B. Dunina, I.A. Khodasevich, V.A. Orlovich, N.V. Kuleshov, Growth and spectroscopic properties of Tm3+:NaBi(MoO4)2 single crystal. Opt. Mater. 80, 169–176 (2018)
M. Yu, W. Zhang, S. Qin, J. Li, K. Qiu, Synthesis and luminescence properties of single-component Ca5(PO4)3F:Dy3+, Eu3+ white-emitting phosphors. J. Am. Ceram. Soc. 101, 4582–4590 (2018)
D. Geng, M. Shang, Y. Zhang, H. Lian, J. Lin, Color-tunable and white luminescence properties via energy transfer in single-phase KNaCa2(PO4)2:A (A = Ce3+, Eu2+, Tb3+, Mn2+, Sm3+) phosphors. Inorg. Chem. 52, 13708–13718 (2013)
K. Wang, Y. Liu, G. Tan, D. Liu, S. Ma, M. Zhao, Structure, luminescence and energy transfer of LiLa(MoO4)2:Dy3+, Eu3+ crystal. J. Lumin. 197, 354–359 (2018)
D. Rajesh, K. Brahmachary, Y.C. Ratnakaram, N. Kiran, A.P. Baker, G.-G. Wang, Energy transfer based emission analysis of Dy3+/Eu3+ co-doped ZANP glasses for white LED applications. J. Alloys Compd. 646, 1096–1103 (2015)
B. Devakumar, P. Halappa, C. Shivakumara, Dy3+/Eu3+ co-doped CsGd(MoO4)2 phosphor with tunable photoluminescence properties for near-UV WLEDs applications. Dyes Pigments 137, 244–255 (2017)
M. Shokouhimehr, S.M. Rafiaei, Combustion synthesized YVO4:Eu3+ phosphors: effect of fuels on nanostructure and luminescence properties. Ceram. Int. 43, 11469–11473 (2017)
T. Li, C. Guo, Y. Wu, L. Li, J.H. Jeong, Green upconversion luminescence in Yb3+/Er3+ co-doped ALn(MoO4)2 (A = Li, Na and K; Ln = La, Gd and Y). J. Alloys Compd. 540, 107–112 (2012)
J. Liu, H. Lian, C. Shi, Improved optical photoluminescence by charge compensation in the phosphor system CaMoO4:Eu3+. Opt. Mater. 29, 1591–1594 (2007)
P. Du, J.S. Yu, Energy transfer mechanism and color controllable luminescence in Dy3+/Eu3+-codoped NaLa(MoO4)2 phosphors. J. Alloys Compd. 653, 468–473 (2015)
R. He, S. Cao, P. Zhou, J. Yu, Recent advances in visible light Bi-based photocatalysts. J. Catal. 35, 989–1007 (2014)
H. Kato, N. Matsudo, A. Kudo, Photophysical and photocatalytic properties of molybdates and tungstates with a scheelite structure. Chem. Lett. 33, 1216–1217 (2004)
A. Kudo, Y. Miseki, Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009)
S. Dutta, S. Som, S.K. Sharma, Luminescence and photometric characterization of K+ compensated CaMoO4:Dy3+ nanophosphors. Dalton Trans. 42, 9654–9661 (2013)
G. Blasse, Energy transfer in oxidic phosphors. Phys. Lett. 28, 444–445 (1968)
L.-L. Wang, Q.-L. Wang, X.-Y. Xu, J.-Z. Li, L.-B. Gao, W.-K. Kang, J.-S. Shi, J. Wang, Energy transfer from Bi3+ to Eu3+ triggers exceptional long-wavelength excitation band in ZnWO4:Bi3+, Eu3+ phosphors. J. Mater. Chem. C 1, 8033–8040 (2013)
F. Kang, X. Yang, M. Peng, L. Wondraczek, Z. Ma, Q. Zhang, J. Qiu, Red Photoluminescence from Bi3+ and the influence of the oxygen-vacancy perturbation in ScVO4: a combined experimental and theoretical study. J. Phys. Chem. C 118, 7515–7522 (2014)
M. Vijayakumar, K. Mahesvaran, D.K. Patel, S. Arunkumar, K. Marimuthu, Structural and optical properties of Dy3+ doped aluminofluoroborophosphate glasses for white light applications. Opt. Mater. 37, 695–705 (2014)
S. Som, P. Mitra, V. Kumar, J.J. Terblans, H.C. Swart, S.K. Sharma, The energy transfer phenomena and colour tunability in Y2O2S:Eu3+/Dy3+ micro-fibers for white emission in solid state lighting applications. Dalton Trans. 43, 9860–9871 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Zhang, W., Li, W. et al. Synthesis and luminescence properties of Eu3+ co-doped NaBi(MoO4)2:Dy3+ phosphors for white light-emitting diodes. J Mater Sci: Mater Electron 30, 658–666 (2019). https://doi.org/10.1007/s10854-018-0334-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0334-0