Abstract
Eu2+/Dy3+ doped SrAl2Si2O8 phosphors were synthesized by a solid state reaction. The phase and luminescent properties of the synthesized phosphors were investigated by the X-ray powder diffraction, photoluminescence spectra, decay curves and the thermo-luminescence glow curves. The XRD results show that the doped Eu2+/Dy3+ has no influence on the phase of SrAl2Si2O8. The incorporation of Dy3+ could significantly enhance the intensity and prolong the afterglow duration of SrAl2Si2O8:Eu2+. The co-doped Dy3+ ions act as trap centers and trap the electrons generated during exposure of the phosphor to an excitation source, which induces the longer afterglow comparing with Eu2+ singly doped SrAl2Si2O8 phosphor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Long afterglow phosphors are a special type of luminescent material with persistent luminescence that lasts for several minutes or hours after the removal of the activated light source. It is generally accepted that the persistent luminescence involves luminescence and trap centers in a long afterglow phosphor [1]. Charge carriers are generated by the excitation in the luminescence centers and subsequently trapped in the trap centers. If the traps are deep, the carriers may reside there for an infinite time when no external stimulation is provided. Their de-trapping is thermally activated, which can cause a delay in the spectral emission and result in the persistent luminescence. Long afterglow phosphors have wide applications in various fields, such as displays, lighting devices, traffic signs, emergency signages, textile printing, decorations and watch dials [2]. Since the first report of SrAl2O4:Eu2+, Dy3+ persistent phosphor [3], rare earth ions doped persistent phosphors have continuously gained popularity. Among various materials, alkaline earth metal (Ca, Sr, Ba) aluminates or silicates have attracted great attention because there are relatively wide band gaps. It has been found that they are suitable for persistent luminescence centers of Eu2+ and Ce3+ [4].
Aluminosilicate compounds have been extensively studied as an important family of host materials for rare earth ions because of their excellently physical and chemical properties, high efficiency and thermal stability [5]. Among various aluminosilicate compounds, (Ca, Sr, Ba)Al2Si2O8 have been reported to act as hosts for various rare earth ions [6,7,8,9,10,11,12]. Moreover, it has been found that it can show long afterglow properties after the dopant of Eu2+ [12]. In this work, we report on the synthesis and long afterglow luminescence of Eu2+/Dy3+ doped SrAl2Si2O8 phosphors. The role of Dy3+ in SrAl2Si2O8:Eu2+ phosphor for the extension of luminescence has been investigated.
2 Materials and methods
SrAl2Si2O8:0.02Eu2+, SrAl2Si2O8:0.02Dy3+ and SrAl2Si2O8:0.02Eu2+/0.02Dy3+ phosphors were synthesized by a solid state reaction. SrCO3 (99.5%), α-Al2O3 (99.5%), SiO2 (99.0%), Eu2O3 (99.99%) and Dy2O3 (99.99%) were used as starting materials. In the synthesis, stoichiometric amounts of raw materials were weighted and mixed thoroughly in an agate mortar by grinding for 60 min. The obtained mixture subsequently was transferred to an alumina crucible and calcined at 1400 °C for 6 h in a muffle furnace. The SrAl2Si2O8:0.02Dy3+ was calcined in air, but SrAl2Si2O8:0.02Eu2+ and SrAl2Si2O8:0.02Eu2+/0.02Dy3+ were calcined under a reducing atmosphere (5% H2/95% N2). After that, the products were cooled to room temperature in the furnace, collected and re-grinded for the next measurements.
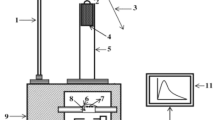
The X-ray diffraction (XRD) patterns were performed on a Rigaku D/max-RA X-ray diffractometer with Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 30 mA. The data were recorded in a 2θ angle of 10°–55° with a scan speed of 10°/min. The excitation and emission spectra were measured by an Edinburgh Instrument FLS920 spectrophotometer equipped with a 150 W xenon lamp as the excitation source and a grating to select a suitable excitation wavelength with excitation and emission slit widths of 2.5 nm, as well as a scan rate of 600 nm/min. Afterglow decay curves were measured by a PR305 long-lasting luminescence detector after the samples were irradiated by ultraviolet light at 365 nm for 10 min. The thermo-luminescence measurements were carried out in the temperature range of 300–500 K with a heating rate of 1 K/s.
3 Results and discussion
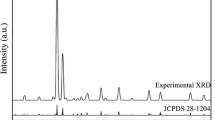
Figure 1 shows the XRD patterns of SrAl2Si2O8:0.02Eu2+, SrAl2Si2O8:0.02Dy3+ and SrAl2Si2O8:0.02Eu2+/0.02Dy3+ phosphors. All of diffraction peaks are well accordance with the standard data of JCPDs 38-1454, indicating that the doped Eu2+/Dy3+ ions have no significant influence on the structure of SrAl2Si2O8 host. There are no diffractions peaks corresponding to other materials, suggesting that Eu2+/Dy3+ ions have doped into SrAl2Si2O8 host entirely. There is only one Sr2+ crystallographic site in the monoclinic SrAl2Si2O8 unit cell and the Sr2+ is bonded to eight oxygen atoms forming an irregular hexahedron, but the Al3+ and Si4+ connect four O atoms to form three-dimensional (Al/Si)O4 frameworks and creates an infinite net structure by corner-sharking. Due to similar ionic radii of Eu2+ (1.25 Å, CN = 8), Dy3+ (1.027 Å, CN = 8) and Sr2+ (1.26 Å, CN = 8), Eu2+ and Dy3+ ions substitute the Sr2+ sites in the synthesized phosphors.
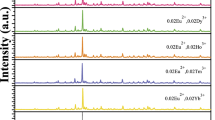
Figure 2 presents the excitation and emission spectra of SrAl2Si2O8:0.02Eu2+. By monitoring the emission at 415 nm, the excitation spectrum of SrAl2Si2O8:0.02Eu2+ exhibits a strong excitation band with a peak at about 365 nm, which results from the 4f7 → 4f6 5d transitions of Eu2+ [13]. Under the excitation at 365 nm, the emission spectrum of SrAl2Si2O8:0.02Eu2+ shows a strong emission band with a peak at about 415 nm, which originates from the spin-allowed transition from the 5d to the 4f state of Eu2+ [4]. Figure 3 gives the excitation and emission spectra of SrAl2Si2O8:0.02Dy3+. The excitation spectrum monitored at 573 nm consists of a series of excitation bands in the range of 300–500 nm with peaks at about 322 nm, 346 nm, 361 nm, 383 nm, 420 nm, 446 nm and 451 nm, which come from the 6H15/2 → 4M17/2, 6H15/2 → 4M15/2, 6H15/2 → 4I11/2, 6H15/2 → 4I13/2, 6H15/2 → 4G11/2 and 6H15/2 → 4G15/2 transitions of Dy3+ [14]. Among these excitation bands, the excitation band peaking at 346 nm is much stronger than other excitation bands. Under the excitation at 346 nm, SrAl2Si2O8:0.02Dy3+ exhibits three emission bands peaking at 480 nm, 573 nm and 664 nm, which originate from the 4F9/2 → 6H15/2, 4F9/2 → 6H13/2 and 4F9/2 → 6H11/2 transitions of Dy3+, respectively [15]. It is known that the yellow emission originating from the 4F9/2 → 6H13/2 transition belongs to a hypersensitive (forced dielectric dipole) transition with the selection rule ∆J = 2, which is influenced by the surrounding environment. However, the blue emission corresponding to the 4F9/2 → 6H15/2 transition is a magnetic dipole transition, which hardly varies with the crystal field around Dy3+ ions. The yellow emission is predominant only when Dy3+ ions locate at low-symmetry sites without inversion centers, but the blue emission will be predominant if the ligand-field deviates from inversion symmetry in the host [16]. The stronger yellow emission indicates that Dy3+ ions are in the low symmetry sites without inversion. That is consistent with coordination surrounding of Sr2+ ions.
Figure 4 exhibits the excitation and emission spectra of SrAl2Si2O8:0.02Eu2+/0.02Dy3+. There is only one excitation band peaking at about 365 nm in the excitation spectrum of SrAl2Si2O8:0.02Eu2+/0.02Dy3+, which does not show any significant changes from the SrAl2Si2O8:0.02Eu2+ except for the higher intensity. And this excitation band is also induced by the 4f7 → 4f6 5d transitions of Eu2+. Under the excitation at 365 nm, SrAl2Si2O8:0.02Eu2+/0.02Dy3+ shows an emission spectrum consisting of a strong emission band with a peak at about 416 nm. The luminescence of SrAl2Si2O8:0.02Eu2+/0.02Dy3+ tells us that there is no energy transfer between Eu2+/Dy3+ ion pairs although the emission spectrum of SrAl2Si2O8:0.02Eu2+ overlaps with the excitation spectrum of SrAl2Si2O8:0.02Dy3+. Figure 5 presents the afterglow decay curves of SrAl2Si2O8:0.02Eu2+ and SrAl2Si2O8:0.02Eu2+/0.02Dy3+. The initial afterglow intensity shows a rapid decay process firstly and then it displays slow decay with lasting decay time. The decay times of SrAl2Si2O8:0.02Eu2+ and SrAl2Si2O8:0.02Eu2+/0.02Dy3+ are about 20.6 min and 33.7 min, respectively. It can be seen that the decay time of SrAl2Si2O8:0.02Eu2+/0.02Dy3+ is longer than that of SrAl2Si2O8:0.02Eu2+, which is induced by the hole traps corresponding to the codoped Dy3+ ions [12]. The co-doped Dy3+ ions act as trap centers and trap the electrons generated during exposure of the phosphor to an excitation source and the afterglow mechanism could be described as producing more traps and stabilizing trapping electrons. On one hand, parts of the divalent alkaline earth cation (Sr2+) were replaced aliovalently by the trivalent rare earth cation (Dy3+) in SrAl2Si2O8. In order to keep charge balance, a strontium vacancy (VSr″) and two DySr sites were generated. This process can be express by the formula of 3SrSr → 2DySr + VSr″ [17]. Therefore, new traps would capture more charge carrying. This result means the concentration of trapped charge will increase. When these carriers were released by thermal agitation at room temperature, SrAl2Si2O8:0.02Eu2+/0.02Dy3+ can emit a stronger afterglow than before. On the other hand, the optical electronegativity of the Dy3+ cation (1.21 eV) is very suitable for stabilizing the captured electrons around traps.
In order to understand the trapping nature of the synthesized phosphors and study the effect of codoped Dy3+ ions in SrAl2Si2O8:Eu2+ on the persistent luminescence process, thermo-luminescence spectra were measured after stopping 365 nm radiations, as shown in Fig. 6. It can be seen that the emission intensity of thermo-luminescence for SrAl2Si2O8:0.02Eu2+/0.02Dy3+ is higher than that of SrAl2Si2O8:0.02Eu2+. The higher thermo-luminescence intensity means that there is more traps in SrAl2Si2O8:0.02Eu2+/0.02Dy3+. As discussed above, the co-doped Dy3+ acts as a trap center and traps the electrons generated during exposure of the phosphor to an excitation source, which induce the higher amount of traps in SrAl2Si2O8:0.02Eu2+/0.02Dy3+. Moreover, the profile of thermo-luminescence spectrum for SrAl2Si2O8:0.02Eu2+/0.02Dy3+ is wider than that of SrAl2Si2O8:0.02Eu2+. The integral area of the thermo-luminescence represents the fact that traps captured the gross amount of charge carriers [4]. The broad thermo-luminescence spectrum covers from 300 to 490 K, which indicates that the traps distribute over a wide range of energies in the bandgap (i.e., traps of various depth were formed).
On the basis of luminescent properties of SrAl2Si2O8:0.02Eu2+ and SrAl2Si2O8:0.02Eu2+/0.02Dy3+, the possible mechanism of persistent luminescence was speculated. On exposure to an excitation source, an electron of Eu2+ (4f7) is promoted to the 4f65d1 band, followed by either direct or phonon-assisted escape of the electron from Eu2+ to the host conduction band. The electrons are subsequently captured by shallow traps. During thermal disturbance at room temperature, captured electrons were released from shallow traps and backtracked to the 5d energy levels via a conduction band. Finally, these electrons recombined with emission centers to generate persistent luminescence. Lattice defects close to the bottom of the host conduction band trap the electrons. Huge numbers of electrons are caught by the traps assisted by Dy3+ at various depths, highlighting an important role for Dy3+ during the long afterglow. After removal of the excitation source, the captured electrons near the host conduction band are released to the conduction band with thermal energy, and their consequent recombination with the emitting Eu2+ centers leads to the persistent afterglow.
4 Conclusions
We synthesized Eu2+/Dy3+ doped SrAl2Si2O8 phosphors through a solid state reaction. Eu2+/Dy3+ singly doped SrAl2Si2O8 phosphor respectively shows characteristic emission bands of Eu2+ or Dy3+, and Eu2+/Dy3+ codoped SrAl2Si2O8 phosphor only exhibits emission band of Eu2+ with higher intensity than that of Eu2+ singly doped SrAl2Si2O8. Both SrAl2Si2O8:Eu2+ and SrAl2Si2O8:Eu2+/Dy3+ show long afterglow luminescence. In SrAl2Si2O8:Eu2+/Dy3+, Dy3+ acts as a trap center and traps the electrons generated during exposure of the phosphor to an excitation source, which induces the longer afterglow comparing with Eu2+ singly doped SrAl2Si2O8 phosphor. The luminescent properties indicate that SrAl2Si2O8:Eu2+/Dy3+ has potential applications in future opto-electronic devices.
References
P. Chandrakar, R.N. Baghel, D.P. Bisen, B.P. Chandra, Luminescence 31, 164 (2016)
I.P. Sahu, J. Mater. Sci.: Mater. Electron. 26, 7059 (2015)
T. Matsuzawa, Y. Aoki, N. Takeuchi, Y. Murayama, J. Electrochem. Soc. 143, 2670 (1996)
P. Wang, X. Xu, D. Zhou, X. Yu, J. Qiu, Inorg. Chem. 54, 1690 (2015)
B. Yuan, Y. Song, Y. Sheng, K. Zheng, X. Zhou, P. Ma, X. Xu, H. Zou, J. Solid State Chem. 232, 169 (2015)
P. Ma, Y. Song, B. Yuan, Y. Sheng, C. Xu, H. Zou, K. Zheng, Ceram. Int. 43, 60 (2017)
P. Ma, B. Yuan, Y. Sheng, K. Zheng, Y. Wang, C. Xu, H. Zou, Y. Song, J. Alloys Compd. 714, 627 (2017)
X. Zheng, Q. Fei, Z. Mao, Y. Liu, Y. Cai, Q. Lu, H. Tian, D. Wang, J. Rare Earth 29, 522 (2011)
J. Chen, Y. Liu, H. Liu, H. Ding, M. Fang, Z. Huang, Opt. Mater. 42, 80 (2015)
Y. Hua, S. Xu, D. Deng, S. Zhao, H. Wang, L. Huang, Opt. Commun. 284, 27 (2011)
H. Xu, L. Wang, M. Li, W. Ran, Z. Deng, R. Houzong, J. Shi, Phys. Status Solidi A 214, 1700013 (2017)
X. Shi, Y. Wang, Z. Wang, P. Zhang, Z. Hong, X. Fan, G. Qian, Acta Photonica Sin. 37, 171 (2008)
M. Zhao, Z. Zhao, L. Yu, L. Yang, J. Jiang, X. Li, G. Li, J. Mater. Sci.: Mater. Electron. 29, 1832 (2018)
X. Tan, Y. Wang, M. Zhang, J. Photoch. Photobio A 363, 65 (2018)
G. Li, J. Mater. Sci.: Mater. Electron. 27, 11012 (2016)
H. Liu, Z. Guo, J. Lumin. 187, 181 (2017)
G. Chen, F. Wang, J. Yu, H. Zhang, X. Zhang, J. Mol. Struct. 1128, 1 (2017)
Acknowledgements
This work is supported by the Basic research Project of Hebei Province (18961031D), the science and technology research project of Hebei’s colleges and university (No. BJ2017035), the Science and Technology Research and Development Program of Hebei Province, Zhangjiakou city (17120011D) and the Doctoral Foundation of Hebei North University (12995557).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, Z., Mao, W., Zhang, K. et al. Enhanced long afterglow of SrAl2Si2O8:Eu2+ by codoping Dy3+. J Mater Sci: Mater Electron 29, 20517–20521 (2018). https://doi.org/10.1007/s10854-018-0188-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0188-5