Abstract
We report the synthesis of magnetite (Fe3O4) and polyvinylpyrrolidone (PVP) coated Fe3O4 nanoparticles by chemical co-precipitation route. X-ray diffraction (XRD) and transmission electron microscope (TEM) confirm the formation of inverse spinel structure of Fe3O4. XRD peaks of PVP coated Fe3O4 nanoparticles are broad and noisy as compared to Fe3O4. The broadness of peaks is due to small size and large defect density confirmed by energy dispersive spectroscopy (EDS) and scattered area electron diffraction (SAED). Noisy behavior is due to presence of PVP. Average particle size reduced from 10.36 ± 1.97 to 6.91 ± 1.89 nm for Fe3O4 and PVP coated Fe3O4, respectively. From EDS analysis, it is confirmed that the oxygen content reduced from 33.45 to 15.30 at.% with PVP coating. The oxygen content is reduced to half in case of PVP coated Fe3O4 as compared to uncoated Fe3O4. The reduction in oxygen content reveals enhancement in oxygen vacancies. Fourier transform infrared (FTIR) spectroscopy and thermogravimetric analysis (TGA) confirm the PVP coating and the calculated value of thickness of the PVP layer on the surface of Fe3O4 is 2.4601 nm. Dielectric constant (εr) and dielectric loss (tanδ) exhibits the dispersion behavior. Ac conductivity (σac) increases sharply at large frequencies, which is due to enhancement in charge density (liberated charge carriers from defects + conduction charge carriers). The variation in dielectric properties and conductivity is due to Maxwell Wagner interfacial polarization and hopping of charge carriers between Fe2+/Fe3+. Magnetic properties M(T) shows reduction of blocking temperature (TB) from 86 to 75 K for uncoated and PVP coated Fe3O4 nanoparticles. Shifting of TB to lower values is consistent with particle size reduction. M(H) loops at room temperature show typical superparamagnetic behavior. Reduction in saturation magnetization (Ms) is due to the presence of nonmagnetic polymer layer on the surface of Fe3O4 nanoparticles and large number of defects (oxygen vacancies). Field cooled M(H) loops at 5 K show the antisymmetric shift of coercive field along the negative x-axis. The exchange bias field HE enhances to 227 Oe in case of PVP coated Fe3O4 nanoparticles, which is double of the 125 Oe for uncoated nanoparticles. The enhancement in HE is due to smaller sized nanoparticles having large surface to volume ratio having large defect density (oxygen vacancies).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnetic fine particles are of great importance as for as their technological applications are considered. Improvements in measuring techniques and synthesis method allowed the preparation of nanostructures at very fine scale [1]. Currently, researchers are synthesizing metallic and oxides particles of very few nanometers in powder form or nanostructures embedded in insulating matrix. Synthesis method play vital role in deciding properties of nanostructures [2,3,4,5]. Magnetic nanostructures have great importance than their bulk counterparts because of their unique properties like superparamagnitism, reduced dielectric losses, smaller value of eddy currents, magnetic saturation at high field and increased anisotropy. Small sized nanoparticles (NPs) have large surface to volume ratio, each particle is reduced to single domain with dominant quantum effects and superparamagnetic properties [3]. Magnetic NPs are widely used in magnetic drug delivery to specific tissues of the body [4], magnetic resonance imaging (MRI) and for diagnosis and treatment of cancerous cells and tumors [5]. Magnetic NPs have also wide range of applications in modern technology such as magnetic storage devices, liquid seals also called ferro fluids (nano sized magnetic grains suspended in carrier liquid) [6].
Among all oxides of iron, magnetite (Fe3O4) and maghemite (γ-Fe2O3) are most important [7]. In most properties of these NPs depend on their synthesis method, which in turn control size, shape and morphology of the particles [8], magnetite nanoparticles are synthesized by wet chemical methods are adopted, which include hydrothermal method, co-precipitation method and sol–gel method [9, 10] etc. Among all these methods, co-precipitation is an appropriate technique to synthesize magnetite NPs. Advantage of this method is that it neither produce any toxic products nor required any complex reactants and the reaction proceeds at room temperature [11]. In modern technology we face challenges of controlling size of NPs, their shapes and dispersibility in desired solvents. Magnetite NPs have large surface to volume ratio and hence large surface energy. This large surface energy makes NPs unstable thermodynamically and results in aggregation of NPs in order to reduce surface energy leading to large sized NPs. Large size (agglomerates) of the magnetite nanoparticles limits their applications. Due to large surface to volume ratio, overall surface area and hence the surface energy of the NPs increases. This enhancement in surface energy results to increase the overall Gibbs free energy and makes nanostructures in general and nanoparticles in particular thermodynamically un-or-metastable. Therefore, NPs have a natural tendency of accumulation to become stable (i.e. in a state of minimum Gibbs free energy). As the dimensions of nanostructured materials reduce, Van der Waals attraction forces between nanostructured materials, becomes increasingly important. Without appropriate stabilization techniques, nanoparticles are most likely and readily to form agglomerates. In order to prevent agglomeration polymeric stabilization is applied to magnetite nanoparticles [12,13,14,15].
Recently, Fe3O4 superparamagnetic nanoparticles are intensely studied because of their wide range of applications in the fields of biology, medicine and material science. Magnetite nanoparticles are often surface-coated for the sake of improvement in their stability and biocompatibility, hydrophilicity and conjugating capability [10, 13]. Coating of Fe3O4 nanoparticles offer many advantages. The magnetic core encapsulated in a protective layer. This layer can be semiconducting, insulating, or metallic [16]. The particles can be well dispersed and the oxidation can be prevented. In case of polymeric protective layer, large number of functional groups of the polymers can be employed around the surface of Fe3O4 nanoparticles to increase functionalization and potentiality [17]. Among all polymers, PVP has certain advantages. It is water soluble, non-charged, non-toxic, excellent dispersant having various applications. Usually, the Fe3O4 nanoparticles tend to form large agglomerates due to their large surface/volume ratio and magnetic dipole–dipole attraction between particles and limit their applications. Polymer coating is an easy way to prevent the agglomeration of Fe3O4 nanoparticles and widen their range of applications.
In this report we synthesized uncoated and polyvinylpyrrolidone (PVP) coated magnetite nanoparticles by chemical co-precipitation method and studied their structural, optical, dielectric and magnetic properties in systematic way.
2 Experimental details
2.1 Materials
Ferrous chloride tetrahydrate (FeCl2·4H2O) 99% and sodium hydro-oxide (NaOH) 99% were purchased from Sigma-Aldrich. Ferric chloride hexahydrate (FeCl3·6H2O) 97.1% and polyvinylpyrrolidone (PVP) (C6H9NO)n 99.5% from Alfa-Aesar were purchased. These reagents were used directly as received. Distilled water was used in the synthesis of nanoparticles.
2.2 Synthesis of magnetite nanoparticles
Polyvinylpyrrolidone (PVP) solution (7 g in 100 ml of distilled water) was heated at 90 °C for 1 h with continuous agitation (1000 rot/min). Then, this PVP solution was added to 350 ml of 1 M NaOH solution. Ferrite solution (50 ml) [containing stoichiometric ratio of 1:2 ferrous chloride tetra hydrate (FeCl2·4H2O) and ferric chloride hexahydrate (FeCl3·6H2O)] was added drop wise to the solution in 30 min (1.6 ml/min). The precipitates were centrifuged and washed with distilled water (6–7 times). The product was separated by centrifugation (6000 rot/min) and dried at 60 °C as per procedure [1, 2]. Then after drying the precipitates are grinded into fine powder by using mortar and pestle. For the synthesis of uncoated magnetite (Fe3O4) procedure is same except the addition of PVP.
The chemical reaction is written as under,
2.3 Materials measurements
The synthesized samples were characterized by X-ray diffraction (XRD) using the XRD Empyrean 200895 (The Netherlands) with Cu Kα radiation with a wavelength λ = 1.5418 Å at 2θ values between 20° and 80°. The morphologies of the products were investigated by a Hitachi S-4800 field-emission scanning electron microscope (FE-SEM) with an accelerating voltage of 5 kV. Transmission electron microscopy (TEM) images were recorded from a transmission electron microscope TEM FEI F20 (USA) system. The chemical composition was analyzed by using an energy dispersive X-ray (EDS) spectroscopy attached to the FE-SEM. Fourier transform infrared spectroscopy (FTIR) spectra were obtained by using the FTIR TENSOR 27 system. The dielectric properties and conductivity as a function of frequency were measured on centered Gold electrode pellet with a typical Impedance analyzer (Agilent 4292) in frequency range (40–15 M) Hz. Dc magnetization [M(H)] measurements were carried out using a Quantum Design magnetic properties measurement system (MPMS) at room temperature.
3 Results and discussion
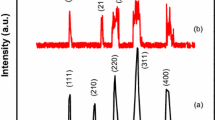
XRD patterns of the virgin and PVP coated magnetite nanoparticles (NPs) are shown in Fig. 1. All the diffraction peaks of the XRD patterns for both the un-coated and PVP coated samples are well matched to the inverse spinel structure of Fe3O4 (JCPDS, file no = PDF # 26-1136) with space group Fd-3m (227) having no impurity peaks, which confirm the formation of above mentioned Fe3O4 structure. Comparison of XRD patterns shows peaks for PVP coated sample are more broadened and less intense than uncoated NPs which is most probably due to presence of PVP on the surface of Fe3O4 NPs. Also enhancement in broadness of peaks is due to decrease in crystallite size and increase in structural disorder [16, 17]. The average crystallite size calculated using Scherer’s formula is 8.7718 ± 0.861 nm and 6.69 ± 2.611 nm for pure and PVP coated magnetite NPs, respectively. Different parameters like plane spacing (\({d_{hkl}}\)), lattice parameters, lattice volume, porosity, and specific surface area are calculated from XRD data and presented in Table 1. Plane spacings are calculated by using Bragg’s law (\(~2{d_{hkl}}\sin \theta =\lambda\)) for each plane. Lattice parameter \(~(a)\) for cubical unit cell is calculated by relation.
where h, k and l are miller indices, and d is plane spacing. Average crystallite size was calculated by using Debye–Scherer’s formula [18].
where \(\beta ,~\)is full width at half maximum and it is calculated in radian, \(\theta\) is angle of plane and \(\lambda\) is wavelength of X-rays used, in present case Cu \({k_\alpha }\) X-rays were used having \(\lambda =1.5406\) Å. For porosity calculation masses of virgin and coated samples for XRD analysis were taken \({m_v}=0.26~\;{\text{g}}~\) and \({m_c}~=0.24~~\;{\text{g}},\) radius of pellet was same for both samples, \({r_v}=~{r_c}=4.5~\;{\text{mm}}\), and heights of pellet were \({h_v}=2.5~\;{\text{mm}}\), and \(~{h_c}=2.1\;{\text{mm}}.\) \(M=231.537\;\;{\text{g/mol}}~\) represents molecular weight of Fe3O4 and \({N_{A~}}\) is Avogadro number. Results showed that PVP coating has decreased lattice parameter (a) from 8.369 to 8.279 Å, which in turn has decreased lattice volume, and average crystallite size reduced from 8.7718 ± 0.861 to 6.69 ± 2.611 nm for uncoated and PVP coated magnetite nanoparticles, respectively.
Figure 2a–d show SEM and EDS analysis of pure and PVP coated magnetite NPs. Figure 2a, b show SEM images of pure and PVP coated magnetite NPs respectively, comparison of both images show that in case of un-coated sample particles have formed dense clusters, they are extensively agglomerated, while in case of SEM image of PVP coated magnetite NPs shows that agglomeration has decreased due to coating and particles are seem to be separated from each other. Figure 2c, d show EDS analysis of pure and PVP coated magnetite NPs. For pure sample peaks are observed only for Oxygen and Iron. Peaks of iron at 0.705 keV, 6.5 keV and 7 keV are due to Lα, Kα and Kβ X-rays respectively. Percent weight composition for iron was 66.55% and for oxygen it is 33.45%. No other element than Fe and O are observed, which indicates that it is pure Fe3O4. In case of PVP (C6H9ON)n coated sample peaks are observed for iron, oxygen and carbon. Presence of carbon peak confirms the PVP layer on the surface of magnetite NPs, hydrogen peaks are not observed in EDS because hydrogen has only single shell so, transition of electron between shells is not possible and it is not detected in EDS. Comparative EDS analysis of both samples shows that atomic percentage of oxygen is reduced from 63.69 to 37.45% due to PVP coating, which means that that concentration of oxygen vacancies increased in case of PVP coated sample than pure sample. These oxygen vacancies play important role in controling different properties of material. Atomic percent of iron is also decreased, which shows presence of iron vacancies in PVP coated sample. The presence of PVP on the surface of magnetite nanoparticles prevents not only agglomeration but also oxidation of the nanoparticles and as a result oxygen content is reduced. These nanoparticle having large specific surface area accomodating large oxygen deficient regions and hence large number of oxygen vacancies are formed.
Figure 3a–f represents TEM images of uncoated and PVP coated magnetite nanoparticles. Figure 3a, b represent spherical shaped uncoated and PVP coated magnetite nanoparticles, respectively. The particle size distribution 5–15 nm with mean particle 10.36 ± 1.97 nm and 4–10 nm with mean particle size of 6.91 ± 1.89 nm for uncoated and PVP coated magnetite nanoparticles as shown in Fig. 3c, d, respectively. It is confirmed that with PVP coating particle size reduced and hence prevents agglomeration of nanoparticles. Figure 3e, f depict the scattered area electron diffraction (SAED) patterns of uncoated and PVP coated nanoparticles. Number of spots is superimposed on diffraction rings of the inverse spinel structure of magnetite nanoparticles and these random spots increased in case of PVP coated magnetite nanoparticles as shown in Fig. 3f. It shows that crystallinity is decreased in case of PVP coated nanoparticles and hence defect density increased. Six fringe patterns of the planes with (hkl) indices of (220), (311), (400), (422), (511) and (440) and their corresponding interplanar distance “dhkl” values are 2.7691 Å, 2.6574 Å, 2.1107 Å, 1.6945 Å, 1.6379 Å and 1.4526 Å are consistent with the peaks observed in XRD and their calculated “dhkl” values [22]. The TEM and XRD results agree firmly well.
FTIR spectra of pure PVP, PVP coated and un-coated magnetite NPs is shown in Fig. 4. Initial peak at 580/cm is due to stretching vibration mode in Fe–O bond both in PVP coated and un-coated magnetite NPs [1]. Peaks at 845 in pure PVP and 857/cm in PVP coated magnetite NPs are attributed to C–C ring stretching. Peaks observed in pure PVP at 1012, 1292 and 1445/cm and in PVP coated sample at 1052/cm, 1340/cm and 1452/cm are attributed to C–N stretching, CH2 bending and CH2 scissoring, respectively [19, 20]. Carbonyl group (C=O) absorb energy at 1661 and 1550/cm in pure PVP and PVP coated magnetite NPs due to its vibrational mode [21]. Peak at 2931/cm is attributed to C–H asymmetric stretching vibration. At the end high intensity broad peak is observed at 3440/cm due to O–H group stretching [21]. Presence of different groups like C–N, O–H, C–H, C–C and C=O confirms the presence of PVP content on the surface of magnetite NPs.
Figure 5 shows TGA curves for pure PVP, un-coated and PVP coated magnetite NPs. Both samples were heated from room temperature to 1000 °C in presence of air. Initially at low temperature mass of all samples slightly increases due to oxidization of Fe ions on the surface to form iron oxides. Mass loss below 200 °C is due to desorption of water molecules, which were physically adsorbed on the surfaces of these particles [22,23,24,25], further decrease in mass of un-coated sample is due to phase changes. At 250 °C it is converted to γ-Fe2O3 and then converted to α-Fe2O3 up to 350 °C, this phase remains constant up to higher temperatures, so no appreciable change in mass is then observed. In case of coated sample more mass loss is observed than virgin magnetite nanoparticles, which is due to burning of PVP on the surface. Pure PVP shows greatest weight loss among all samples because all PVP burns at 675 °C [24].
3.1 Calculation of PVP thickness on magnetite surface
PVP content (thickness of PVP layer on surface of magnetite NPs) can be calculated by % mass decrease. To calculate the thickness of the PVP’s layer, some simplifying assumptions can be made for this purpose first of all it is assumed that particles are spherical in shape. Second, magnetic core is purely magnetite; this assumption has been confirmed by XRD pattern. Third, particles are mono-dispersed. In order to calculate the thickness of PVP layer on surface of spherical Fe3O4, forth assumption is taken that PVP forms spherical layer of thickness (t) on magnetite surface; it is calculated by formula given below [22, 23]
where \({\rho _v}\) and \({{\text{m}}_v}\) are density and mass of un-coated (virgin) magnetite NPs, R is radius of PVP coated magnetite. Similarly \({\rho _c}\) and \(~{{\text{m}}_c}~\) are density and mass of PVP coated magnetite NPs. Initial mass of un-coted sample was taken 4.130 mg and % decrease in mass of this sample was 9.2437%. For PVP coated sample initial mass was taken mc = 14.7081 mg and % decrease in this sample was 17.3393%, in which 9.2437% decrease is due to burning of PVP.
At 675 °C all PVP (ρc = 1.67 g/cm3) structurally decomposed and remaining is un-coated magnetite (which was core initially) having density ρv = 5.26 g/cm3. The mass of the sample at this temperature was mv = 12.1578 mg. From TEM result, PVP coated NPs have an average particle size of 6.91 nm, which corresponds to radius R = 3.45 nm. Putting all these values in above formula, calculated value of thickness of PVP’s layer on magnetite NPs surface was 2.4601 nm.
Figure 6 shows changes in dielectric constant with frequency for both uncoated and PVP coated nanoparticles. Reduced dielectric constant for PVP coated sample as compared to uncoated (pure) nanoparticles can be seen, which is due to presence of polymer layer on surface of NPs as confirmed by TGA, EDS and FTIR. Overall behavior in case of both samples shows decrease in dielectric constant with enhancement in frequency. Change in dielectric constant \(~{\varepsilon _r}\), with change in frequency is termed as dielectric dispersion behavior. Both samples exhibit dielectric dispersion behavior in low frequency range. Large values of dielectric constant at low frequencies are due to electronic polarization, which contributes at these frequencies. Interfacial polarization is also dominant in lower frequencies region in nanomaterial due to large surface to volume ratio. On the basis of Maxwell–Wagner theory of interfacial polarization [15, 25] along with Koop’s phenomenological theory [26, 27], small sized nanoparticles have large number of grains and grain boundaries. Grains are conducting and grain boundaries are resistive. So charge carriers move in the direction of applied field up to the grain boundaries, due to resistive nature of grain boundaries further movement of charge carriers cannot take place and accumulation of charge carriers results in large polarization at low frequencies. According to Rezlescue model [28] hopping process of charge carriers takes place between Fe2+/Fe3+ ions for dielectric polarization [29]. At higher frequencies dielectric constant is independent of frequencies because all modes of polarizations, contributing to dielectric constant lags the frequency of applied field [30,31,32,33].
Dielectric loss tangent (tan δ) at room temperature, as function of frequency for PVP coated and un-coated magnetite NPs is shown in Fig. 7. It is clearly observed that loss tangent decreases with enhancement in frequencies for both uncoated and PVP coated NPs. Two mechanisms electron hopping between Fe2+/Fe3+ and defect dipoles are considered as cause of dielectric loss in ferrites [15, 33,34,35]. High dielectric loss in low frequencies region is due to hopping of charge carriers which increases polarization, so more energy is lost when dipoles changes direction in applied field. At large frequencies defect dipoles decreases dielectric loss. Charges could not keep up with frequencies of external field at large frequencies and as a result dielectric loss decreases. No relaxation peak is observed for both samples [36].
Variation of conductivity with change in frequency for uncoated and PVP coated magnetite NPs is shown in Fig. 8. In low frequencies region, it has been observed that conductivity is very low and independent of frequencies, it increases with enhancement in frequency and then sharp increase in conductivity is observed at large frequencies. Nonlinear behavior of conductivity with frequency is due to large polarons (their radius is greater than lattice parameters) [36]. These variations in conductivity with changing frequency can be well explained on the basis hopping and Koop’s model [26, 27]. According to Koop’s model grain boundaries play important role in controlling conductivity. In nanomaterial as the size of NPs decreases surface to volume ratio increases hence number of interfaces increases, it is reported that nanomaterial have 1019 interfaces/cm3 [38,39,40], so this high density of interfaces provide charge carriers due to ionization as well as serve as conduction centers for transport of charges and hence increases conductivity of nanomaterials as compare to their bulk counterparts [39]. Low conductivity at lower frequency regime is due to poor conducting grain boundaries according to Koop’s model, enhancement in conductivity with increasing frequency can be explained on the basis of hopping model, hopping of electrons between Fe2+/Fe3+ ions increases with increasing frequency which increases conductivity. The observed dispersion of conductivity at higher frequencies is due to conducting grains at these frequencies [22, 40]. Reduced conductivity of PVP coated sample than un-coated sample may be the result of reduced size having more grain boundaries than uncoated sample. The increase in conductivity at higher frequencies is due to increased charge density. Large concentration of oxygen vacancies trap charge carriers which are set free at high frequencies of applied field and as result (liberated charge carriers + conduction charge carriers) increases charge density and hence conductivity [39, 40].
Figure 9 shows room temperature M(H) loops for both uncoated and PVP coated samples. Both samples show superparamagnetic behavior a common characteristic of single domain particles of very small dimensions. The saturation magnetization for uncoated nanoparticles is 39.5 emu/g, while for PVP coated NPs it is 29.97 emu/g. The reduction in magnetization in case of PVP coated nanoparticles is most probably due to large number of defects (oxygen vacancies, cation vacancies) on the surface of small sized NPs and the existence of a non-magnetic polymer (PVP) layer on the surface of particles [22, 41].
The M(T) [zero field cooled (ZFC) and field cooled (FC) magnetization] measurements for both coated and uncoated samples are shown in Fig. 10. In ZFC measurement sample is cooled from room temperature to 5 K. In case of FC 1 kOe field was switched on at room temperature (300 K) and magnetization data was recorded while cooling the sample up to 5 K. In case of PVP coated sample reduction in magnetization and shifting of blocking temperature TB towards lower temperature values are noted. Reduction in magnetization is due the presence of a non-magnetic layer of polymer (PVP) on the surface of nanoparticles and large number of oxygen vacancies. The values of blocking temperature for both uncoated and PVP coated magnetite are 86 and 75 K, respectively. The shifting of blocking temperature TB towards the lower temperature values is due to the polymer (PVP) coating, which may have reduced the inter-particle dipole–dipole interactions and possibly lowered the energy barrier for thermal fluctuations and consequently decreased the blocking temperature [41]. Above the blocking temperature TB the particles show superparamagnetic behavior and below blocking temperature TB it shows ferromagnetic behavior. This result is in firm agreement with XRD, SEM and TEM.
The FC M(H) loops for uncoated and PVP coated magnetite are shown in Fig. 11. These particles are called core–shell nanoparticles. The interaction between two different phases (core and shell) at the interface results in the shifting of the center of a hysteresis loop away from the H = 0 point as shown in the inset of Fig. 11. Exchange bias is defined as the difference of the absolute values of the positive (Hc2) and negative coercive (Hc1) fields as given in Eq. (4).
where \({{\text{H}}_{\text{E}}}\) is called the exchange field. Two FC M(H) loops (cooled in a 1 kOe applied field) are shown in Fig. 11 for the comparison of exchange bias HE in uncoated and PVP coated samples. The exchange bias field HE is due to existence of a magnetically disordered surface layer (shell) that becomes frozen below the blocking temperature TB = 75 K [42]. The observed enhancement in the coercivity (shift of negative coercive field) for switching the core spins that are pinned by the exchange interactions with the frozen shell spins (spin glass-like layer at the surface). An increase in exchange bias field HE is observed in PVP coated sample 227 Oe as compared to uncoated sample 125 Oe as shown in inset of Fig. 11 [16, 41, 42]. Exchange bias originates from the exchange coupling between core ferromagnetic (ordered) spins and shell antiferromagnetic (disordered) frozen spins. The saturation magnetization Ms and remanent magnetization Mr are Ms = 35 emu/g, Mr = 12 emu/g and Ms = 51 emu/g, Mr = 10 emu/g, respectively. The reason of enhanced exchange bias in coated sample is large defect density (oxygen vacancies) and reduced size (larger surface to volume ratio) (as confirmed by particle size reduction, clear from XRD, SEM and TEM results) as compared to uncoated sample, leading possibly to a larger surface area and hence a higher surface spin disorder. These effects may collectively results in a larger exchange anisotropy or bias [16, 42]. The problem of superparamagnetic can be overcome by increased coercivity when particles are coated. PVP coating may result in the reduction of exchange interactions, which results in the increased exchange bias effect. Exchange bias originates from the exchange coupling between core ferromagnetic ordered spins and shell antiferromagnetic (disordered) frozen spins, respectively.
4 Conclusion
Polyvinylpyrrolidone (PVP) coated and uncoated Fe3O4 nanoparticles are synthesized by chemical co-precipitation route. X-ray diffraction (XRD) reveals the inverse spinel structure of both coated uncoated nanoparticles also confirmed by transmission electron microscope (TEM) also. XRD peaks of the PVP coated nanoparticles broad and noisy as compared to the uncoated nanoparticles. Noisy behavior is due to presence of polymer (PVP) and enhancement in broadness is due to reduction in particle size and enhancement in defect density (oxygen vacancies). TEM shows spherical shaped particles with average particle size of 10.36 ± 1.97 nm for Fe3O4 and 6.91 ± 1.89 nm for PVP coated Fe3O4. Energy dispersive X-ray spectroscopy (EDS) shows that the present elements are “Fe” and “O” for uncoated and “Fe”, “O” and “C” for PVP coated nanoparticles. Oxygen content in PVP coated Fe3O4 nanoparticles is half (15.30 at.%) as compared to the oxygen content (33.45 at.%) of uncoated nanoparticles resulting in large number of oxygen vacancies as compared to the uncoated nanoparticles. Fourier transform infrared (FTIR) spectra and thermogravimetric analysis (TGA) confirmed the presence of PVP on the surface of Fe3O4 nanoparticles and the calculated thickness of PVP layer on the surface of Fe3O4 nanoparticles is 2.46 nm. Dielectric constant (εr), conductivity (σac) and dielectric loss (tanδ) show the dispersion behavior which is very well explained by Maxwell Wagner model and hopping model. Magnetic properties M(T) shows shifting of the blocking temperature (TB) to lower temperature values from 86 to 75 K for uncoated and PVP coated samples. Above TB magnetite nanoparticles show superparamagnetic behavior and below TB these nanoparticles show ferromagnetic behavior. The shifting of blocking temperature to lower temperature values is the indication that the inter-particle dipole–dipole interactions are reduced, which most probably lowered the energy barrier for thermal fluctuations and with PVP coating particle size reduced. Room temperature M(H) loops shows typical superparamagnetic behavior and the reduction of saturation magnetization “Ms” from 39.5 to 29.97 emu/g for uncoated and PVP coated Fe3O4 nanoparticles is due to presence of nonmagnetic polymer layer and large number of oxygen vacancies. Exchange bias field (HE) enhanced to 227 Oe in PVP coated nanoparticles as compared to uncoated Fe3O4 nanoparticles. The increase in HE is due to large number of oxygen vacancies and small sized nanoparticles having large surface area in case of PVP coated nanoparticles. This proves that coating is a very useful technique to overcome the problem of superparamagnitism.
References
S.C. Watawe, U.A. Bamane, S.P. Gonbare, R.B. Tangoli, Mater. Chem. Phys. 103, 323 (2007)
R.G. Kharabe, R.S. Devan, C.M. Kanamadi, B.K. Chougule, Smart Mater. Struct. 15, 125 (2006)
X. Batlle, A. Labarta, J. Phys. D Apply. Phys. 35, R15 (2002)
K.J. Lee, J.H. An, J.S. Shin, D.H. Kim, C. Kim, H. Ozaki, J.G. Koh, J. Nanotechnol. 18, 465201 (2007)
P. Tartaj, M.P. Morales, S.V. Verdaguer, T.G. Carreño, C.J. Serna, J. Phys. D Appl. Phys. 36, R182–R197 (2003)
J.M.D. Coey, Phys. Rev. Lett. 27, 1140 (1971)
B. Martínez, X. Obradors, L. Balcells, A. Rouanet, C. Monty, Phys. Rev. Lett. 80, 181 (1998)
C.C. Berry, A.S.G. Curtis, J. Phys. D 36, R198 (2003)
G.F. Goya, T.S. Berquo, F.C. Fonseca. M.P. Morales, J. Appl. Phys. 94, 3520 (2003)
P.P. Sahay, R.K. Mishra, S.N. Pandey, S. Jha, M. Shamsuddin, Curr. Appl. Phys. 13, 479–486 (2013)
N.S. Kommareddi, M. Tata, V.T. John, G.L. Pherson, M.F. Herman, Y.S. Lee, C.J. O’Connor, J.A. Akkara, D.L. Kaplan, Chem. Mater. 8, 801 (1996)
N. Fauconnier, J.N. Pons, A. Roger, Bee, Colloid Interface Sci. 194, 423 (1997)
A.D. Sheikh, V.L. Mathe, J. Mater. Sci. 43, 2018 (2008)
P.P. Hankare, R.P. Patil, U.B. Sankpal, S.D. Jadhav, P.D. Lokhande, K.M. Jadhav, R. Sasikala, Solid State Chem. 182, 3217 (2009)
P.P. Hankare, U.B. Sankpal, R.P. Patil, A.V. Jadhav, K.M. Garadkar, B.K. Chougule, J. Magn. Magn. Mater. 323, 389–393 (2011)
M.P. Morales, S.V. Verdaguer, M.I. Montero, C.J. Serna, Chem. Mater. 11, 3058 (1999)
Zulfiqar, M.U. Rahman, M. Usman, S.K. Hasanain, Z.U. Rahman, A. Ullah, W. Kim, J. Korean Phys. Soc. 65, 1925–1929 (2014)
G.F. Goya, M.P. Morales, J. Metastabl. Nanocrystall. Mater. 673, 20–21 (2004)
D. Cullity, Elements of X-ray Diffraction, 2nd ed. (Addison-Wesley Publishing Inc, Boston, 1978)
X. Lu, L. Li, W. Zhang, C. Wang, J. Nanotechnol. 16, 2233–2237 (2005)
N. Arsalani, H. Fattahi, M. Nazarpoor, Express Polym. Lett. 4, 329–338 (2010)
Zulfiqar, R. Khan, M.U. Rahman, Z. Iqbal, J. Mater. Sci. Mater. Electron. 27, 12490–12498 (2016)
I.M. Mirza, A.K. Sarfraz, S.K. Hasanain, Effect of surfactant on magnetic and optical properties of α-Fe2O3 nanoparticles. ACTA Phys. Pol. A 126, 1280–1287 (2014)
C.J. O’Connor, Y.S.L. Buisson, S. Li, S. Banerjee, R. Premchandran, T. Baumgartner, V.T. John, G.L. McPherson, J.A. Akkara, D.L. Kaplan, J. Appl. Phys. 81, 4741 (1997)
K.W. Wagner, Ann. Phys. 40, 817 (1913)
G.C. Koops, Phys. Rev. 83, 121 (1951)
P.A. Miles, W.B. Phal, A.V. Hippal, Rev. Mod. Phys. 29, 279 (1957)
N. Rezlescu, E. Rezlescu, Phys. Status Solidi A 23, 575 (1974)
D. Ravinder, K. Latha, J. Appl. Phys. 75, 6118 (1994)
A.M. Abdeen, J. Magn. Magn. Mater. 192, 121 (1999)
J.H. Nam, H.H. Jung, J.Y. Shin, J.H. Oh, IEEE Trans. Magn. 31, 3985 (1995)
S.R. Murthy, J. Mater. Sci. Lett. 3, 1049 (1984)
P.P. Hankare, R.P. Patil, U.B. Sankpal, S.D. Jadhav, I.S. Mulla, K.M. Jadhav, B.K. Chougule, J. Magn. Magn. Mater. 321, 3270 (2009)
K.P. Thummer, H.H. Joshi, R.G. Kulkarni, J. Mater. Sci. Lett. 18, 1529 (1999)
B.K. Kunar, P.K. Singh, P. Kishan, N. Kumar, G.P. Srivastava, J. Appl. Phys. 63, 3780 (1988)
S. Mehraj, M.S. Ansari, Alimuddin, Physica E Low Dimens. Syst. Nanostruct. 65, 84–92 (2015)
D. Alder, J. Feinleib, Phys. Rev. B 2, 3112 (1970)
E.V. Gopalan, K.A. Malini, S. Saravanan, D.S. Kumar, Y. Yoshida, M.R. Anantharaman, J. Phys. D 41, 185005 (2008)
M.G. Chourashiya, J.Y. Patil, S.H. Pawar, L.D. Jadhav, Mater. Chem. Phys. 109, 39 (2008)
A.M. Feroz, M.B. Khalid, C. Indrajeet, G.M. Bhat, J. Mater. Sci. Mater. Electron. 25, 1564–1570 (2014)
H. Wang, X. Qiao, J. Chen, X. Wang, S. Ding, Mater. Chem. Phys. 94, 449 (2005)
S. Gangopadhyay, G.C. Hadjipanayis, B. Dale, C.M. Sorensen, K.J. Klabunde, V. Papaefthymiou, A. Kostikas, Phys. Rev. 45, 9778 (1992)
Acknowledgements
This work is financially supported by the Higher Education Commission of Pakistan under START-UP RESEARCH GRANT PROGRAM (Grant no.: 21-1732/SRGP/R&D/HEC/2017), the Fundamental Research Funds for the HEC Pakistan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zulfiqar, Afzal, S., Khan, R. et al. Structural, optical, dielectric and magnetic properties of PVP coated magnetite (Fe3O4) nanoparticles. J Mater Sci: Mater Electron 29, 20040–20050 (2018). https://doi.org/10.1007/s10854-018-0134-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0134-6