Abstract
The development of NO2 gas sensor with high sensitivity, low detection limit and high selectivity is highly required. This article reports a NO2 gas sensor based on Co3O4/In2O3 heterojunction structure fabricated by a two-step hydrothermal method. Particularly, morphological and structural analysis of the Co3O4 nanorods/In2O3 nanocubes nanocomposite was examined by SEM, TEM, XRD, EDS and XPS measurements. The Co3O4/In2O3 nanocomposite sensor was tested toward NO2 gas (1–200 ppm) under different operation temperature. The sensor exhibited excellent gas sensing properties for NO2 sensing at an optimal temperature of 150 °C. The corresponding response is 27.9 to 10 ppm NO2 at 150 °C, 1.2 times higher than that of pure In2O3 and 10 times higher than that of pure Co3O4. Moreover, the Co3O4/In2O3 sensor shows sub-ppm level detection ability, good selectivity and long-term stability at low temperature. The enhanced sensing performance can be attributed to the Co3O4/In2O3 heterojunction structure formed at the interfaces of n-type In2O3 nanocubes and p-type Co3O4 nanorods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As well known, nitrogen dioxide (NO2) gas is a component of atmospheric contaminants and has a threat to human health [1,2,3,4]. High performance NO2 gas sensors have been widely investigated in recent years due to the increasing concerns of living quality and air pollution [5, 6]. To date, a variety of materials have been used to prepare NO2 gas sensors, such as graphene and various metal oxides [7, 8]. Among them, metal oxide semiconductors such as In2O3, ZnO, SnO2 and TeO2 have attracted significant interest due to their unique physical–chemical properties [9,10,11,12].
Among the above materials, In2O3 is reported as a promising n-type semiconductor material, has high sensitivity and good selectivity for NO2 sensing [13,14,15,16,17]. Compared with ZnO, SnO2 and Fe2O3, In2O3 has wider band gap and smaller resistivity [18, 19]. Therefore, the In2O3 semiconductor based NO2 sensors have attracted extensive attention in the last few years. Yan et al. prepared a low temperature NO2 gas sensor based on reduced graphene oxide (rGO)-doped In2O3 composite. The rGO-In2O3 sensor demonstrated excellent sensing properties due to the formation of p–n heterojunctions [20]. Ding et al. synthesized Ag–In2O3 nanostructure for NO2 detection, and achieved enhanced selectivity and high stability [21]. In addition, Kim et al. found that SnO2 core and In2O3 shell structure can boost the sensitivity and response to NO2 detection [22]. Therefore, the noble metal or metal oxide decoration with In2O3 is an alternative route to construct an efficient material for high-performance NO2 sensing [23,24,25,26].
In fact, the construction of heterojunction structure by combining n- and p-type semiconductors to improve the gas sensing performance of a single semiconductor has been widely reported [27,28,29,30,31]. As a p-type semiconductor, Co3O4 has a band gap of 2.2 eV, which can create a heterojunction with the n-type In2O3 [32,33,34,35,36]. However, few studies have been reported on the NO2 sensing properties of Co3O4/In2O3 heterojunction. In this paper, we prepared Co3O4/In2O3 composite nanomaterial using hydrothermal method. To test the as-prepared samples, their morphologies, microstructures, and compositions were systematically characterized by various means consist of XRD, EDS, SEM, TEM and XPS. The gas-sensing measurement of the Co3O4/In2O3 heterostructure sensor shows superior sensitivity and lower detection limit toward NO2, which surpasses that of pristine In2O3 and Co3O4 sensors. At last, the sensing mechanism of the Co3O4/In2O3 heterostructure toward NO2 was discovered.
2 Experimental
2.1 Materials
The chemicals of indium nitrate [In(NO3)3·4.5H2O], cobaltous nitrate [Co(NO3)2·6H2O], urea [CO(NH2)2], trisodium phosphate dodecahydrate (Na3PO4·12H2O), hydrazine hydrate (N2H4·H2O), terpineol and methyl cellulose were obtained from Sinopharm Chemical Reagent.
2.2 Sample fabrication
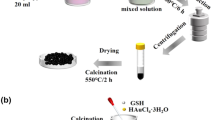
In a typical fabrication of In2O3, 1.75 g In(NO3)3·4.5H2O and 15 g urea were dissolved into 36 mL deionized water. After stirring for 2 h and ultrasonic treating for 50 min, the mix solution was transferred into a 60 mL polytetrafluoroethylene (PTFE) reactor and heated at 120 °C for 12 h. Then the prepared precipitate was centrifuged at 8000 rpm for 5 min, washed with deionized water for several times to remove excess impurity ions, and dried at 80 °C to obtain some In2O3 powders, followed by being calcined in a muffle furnace at 500 °C for 2 h at a heating rate of 2 °C min−1. Similarly, for the fabrication of Co3O4/In2O3 nanocomposite, 0.58 g Co(NO3)2·6H2O and 0.56 g In2O3 nanopowders were ground in an agate mortar to mix the two reagents evenly. The mixture and 20 mg Na3PO4·12H2O were dissolved into 60 mL deionized water, and then stirred and shook for 30 min. Next, 8 mL N2H4·H2O was added into the resulting solution and ultrasonic shook for 30 min, followed by transferring into a 100 mL PTFE reactor and heating at 180 °C for 12 h. The obtained solution was washed several times to remove excess ions, dried at 80 °C to obtain In2O3 powders, and then calcined in a muffle furnace at 450 °C for 2 h. Finally, the black In2O3/Co3O4 nanocomposite was collected and mixed with terpineol and methyl cellulose to form a paste through slight milling. The resulting paste was coated on a ceramic tube as sensing material for NO2 sensing. The ceramic tube has a length of 4 mm and an outer diameter of 1.2 mm. Before testing, the sensing device was aged in air at 300 °C for 24 h before testing.
2.3 Experimental setup
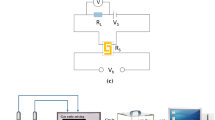
As is seen in Fig. 1, the NO2 gas sensing properties of the fabricated Co3O4/In2O3 nanocomposite were determined by an Agilent 34970A coupled with a computer. The sensor was welded to a six-foot base and placed in a conical flask. Different concentrations of NO2 were introduced to the conical flask through a microsyringe. The operation temperature for the sensor (50–300 °C) was adjusted by applying a voltage to a nickel–chromium heating coil, which is located at the center of ceramic tube. The response is defined as the ratio of the sensor resistance in air and NO2 gas.
3 Results and discussion
3.1 Sample characterization
The crystal structure of the synthesized materials was characterized using XRD (Rigaku D/Max 2500PC). The XRD patterns of the In2O3, Co3O4, and Co3O4/In2O3 samples are shown in the Fig. 2a. The diffraction peaks of In2O3 observed at 2θ = 21.50°, 30.58°, 35.47°, 45.69°, 51.04°, and 60.68° can be ascribed to the (211), (222), (400), (431), (440) and (622) planes of the cubic phase of In2O3 (JCPDS card 04-0614), respectively [37]. The diffraction peaks of Co3O4 sample are observed at 2θ = 31.27°, 36.85°, 44.81°, 59.35°, and 65.23°, which are indexed to the (220), (311), (400), (511) and (440) planes of the cubic phase of Co3O4 (JCPDS card 43-1003), respectively [38]. In the XRD of Co3O4/In2O3 sample, the characteristic peaks In2O3 can be clearly observed due to its high intensity. Nevertheless, the typical diffraction peaks of Co3O4 are not obvious, which may be attributed to the low content of Co3O4 crystals. The elements composition of the Co3O4/In2O3 sample was analyzed by Hitachi S-4800 equipped with an energy-dispersive spectrometer (EDS) [39], as shown in Fig. 2b. It is clearly observed that the elements O, In, and Co exist in the Co3O4/In2O3 heterostructure. No other element has been detected.
The surface morphology of the prepared samples was characterized by a scanning electron microscopy (SEM, Hitachi S-4800), and the SEM images are shown in Fig. 3. The SEM image of In2O3 shown in Fig. 3a indicates nanocube-shaped structure with side length of 250–300 nm. Figure 3b shows the Co3O4 has nanorod-like morphology with length of 1–1.5 µm. Figure 3c, d show that Co3O4/In2O3 is consisted of cubic In2O3 and rod-shaped Co3O4 with good bonding.
Figure 4 shows the TEM images of the In2O3, Co3O4 and Co3O4/In2O3 samples. Figure 4a shows the TEM image of In2O3 nanocubes. Figure 4b shows the TEM image of Co3O4 nanorods. Figure 4c illustrates that the In2O3 nanocubes and Co3O4 nanorods contacted each other in the Co3O4/In2O3 nanocomposite. Figure 4d shows the HRTEM image of the Co3O4/In2O3 nanocomposite. As labeled in Fig. 4d, the lattice fringes with spacing of 0.291 nm and 0.243 nm are indexed to (222) plane of In2O3 nanocube and (311) plane of Co3O4 nanorod, respectively [40,41,42].
Figure 5 shows the XPS spectra of Co3O4/In2O3 nanocomposite. The survey spectrum of Fig. 5a shows the peaks of C, O, In, Co elements without any other impurity elements. The In 3d spectrum of Fig. 5b exhibits In 3d5/2 peak at 441.1 eV and In 3d3/2 peak at 451.7 eV, demonstrating the presence of In2O3. Figure 5c shows that the Co 2p spectrum can be divided into six components with binding energy at 779.3 eV, 783.5 eV, 786.8 eV, 794.5 eV, 796.3 eV and 803.5 eV. As shown in Fig. 5d, the O 1s spectrum has two components, the peaks at 531.60 eV and 529.55 eV can be ascribed to the oxygen species on the sensing film.
3.2 NO2-sensing properties
As well known, the NO2 gas sensing performances of the Co3O4/In2O3 heterojunction, pure In2O3, and Co3O4 film sensors are dependent on the operation temperature. The optimal working temperature was explored by applying adjustable voltages to the sensors for yielding the operation temperature of 50–300 °C. As shown in Fig. 6, the response values of the three sensors upon exposure to 250 ppb NO2 gas increased with the increasing of the operation temperature, and reached a maximum at 150 °C. Afterwards, the response values of the sensors decreased as the temperature further increase. This result can be ascribable to the gas adsorption and desorption properties of the material surfaces at different temperature. When the sensors are exposed to NO2 at room temperature, a small amount of NO2 molecules are adsorbed on the surface of the sensing material. As the operation temperature imposed from 50 °C to 150 °C, much more energies are provided for thermal reactions of NO2 molecules with O2, resulting in a higher response. However, as the temperature increases further, the desorption rate of NO2 gas molecules exceeds the adsorption rate, which depressed the sensor response. The following experiments were conducted at the optimal operating temperature of 150 °C. The response values of the Co3O4/In2O3 sensor toward 250 ppb NO2 is much higher than that of pure In2O3 and Co3O4 sensor, indicating the Co3O4 doped In2O3 is an efficient route to enhance the sensing properties of pure In2O3. The synergistic effects ensure the response of Co3O4/In2O3 heterojunction superior to that of pure In2O3 and Co3O4 for NO2 detection. The heterojunction structure of Co3O4/In2O3 exhibits n-type conducting behavior, which may be owing to high content of In2O3 in the composite material.
Figure 7a shows the sensing performance of pure In2O3, Co3O4 and Co3O4/In2O3 sensors tested toward the NO2 concentration ranging from 1 to 200 ppm. With the rising NO2 concentration, the response values of pure In2O3 and Co3O4/In2O3 sensors increased rapidly. The response value of Co3O4/In2O3 sensor are about 4.6, 20.0, 28.0, 29.8, 64.4, 89.8, 106.8 and 117.2 toward 1, 5, 10, 30, 50, 100 and 200 ppm of NO2 gas, respectively. The response value of the Co3O4 sensor was relatively low. Figure 7b shows the fitting equations between the response of the Co3O4/In2O3 sensor and NO2 concentration can be represented as Y = 118.03(1−0.97X). The Co3O4/In2O3 sensor exhibits an exponential relation with NO2 concentration ranging from 1 to 200 ppm.
The selectivity of the Co3O4/In2O3 sensor for NO2 gas is investigated against the other commonly encountered gases such as H2S, NH3, SO2, CO and CH4, as shown in Fig. 8a. When the Co3O4/In2O3 sensor is exposed to the above six gas species at the same concentration of 10 ppm, the response value of the sensor to NO2 is much higher than that of other gases, which proved an excellent selectivity of the Co3O4/In2O3 sensor. As is well known, good stability and repeatability are important for NO2 gas sensors in practical applications. The repeatability of the Co3O4/In2O3 sensor toward 1 ppm, 5 ppm and 10 ppm of NO2 is shown in Fig. 8b. The response value is basically invariant when the sensor exposed to a given concentration for several times. Figure 8c shows the stepping cumulative response of the Co3O4/In2O3 sensor under the continuous increase of NO2 concentration. We can find the sensor exhibits graded changing trend along with the increased NO2 concentration [43], indicating the sensor has good follow-up tracing ability to the changing concentration. Figure 8d shows the long-term ability of the Co3O4/In2O3 sensor to detect 1, 5 and 10 ppm NO2. There is no significant decrease or increase observed for the sensor operated in a month.
Table 1 presents the comparative analysis of NO2 sensor based on various sensing materials and fabrication techniques. The NO2 gas sensors in terms of measuring range, operation temperature and response are compared between this work and the reported works [44,45,46,47,48,49]. We can find that, the Co3O4/In2O3 film has demonstrated to be an efficient sensing material for constructing low-temperature NO2 sensor.
3.3 NO2-sensing mechanism
Co3O4 is a typical p-type semiconductor, with holes as its main carrier, while In2O3 is an n-type semiconductor with electrons as its main carrier [50,51,52]. Figure 9a shows the mechanism of the In2O3/Co3O4 sensor toward NO2 sensing. When In2O3 is exposed to ambient air at operation temperature, oxygen molecules will be adsorbed onto the surface of In2O3 and trap electrons from its conduction band to form negatively adsorbed oxygen (O2−, O− and O2−) [53], and thus produce an electron depletion layer on its surface. When In2O3 is exposed to NO2 gas, NO2 gas molecules react with the negatively adsorbed oxygen and further capture electrons from the conduction band, resulting in the resistance of In2O3 increase. The reaction equations are described as Eqs. (1–3) [54]:
The synergic effect of In2O3 nanocubes and Co3O4 nanorods contributes a lot to the enhanced NO2 sensing properties. The n-type semiconductor In2O3 has a higher Fermi level than the p-type semiconductor Co3O4, the electrons on In2O3 nanocubes will transfer to Co3O4 nanorods, and the holes will transfer from Co3O4 nanorods to In2O3 nanocubes until the Fermi level reach balance. Therefore, an electron depletion layer is formed at the interface between In2O3 nanocubes and Co3O4 nanorods. As shown in Fig. 9b, when NO2 molecules are introduced to the Co3O4/In2O3 heterostructure, they react with ionized oxygen species and extract electrons, resulting in the increase of depletion layer width and the resistance of Co3O4/In2O3 further increases [55]. Due to the built-in potential, a potential barrier was established at Co3O4/In2O3 and In2O3/In2O3 interfaces. The potential barrier changed between the Co3O4/In2O3 interfaces (p-n junction) is much larger than that of In2O3/In2O3 interfaces. The resistance related to heterojunction barrier can be expressed by Eq. (4) [56]:
where R and R0 are the resistance and initial resistance of the heterojunction barrier, q is the charge of electron, E is the heterojunction potential barrier, k is the Boltzmann’s constant and T is the absolute temperature. Because R depends on the potential barrier (E) at the Co3O4/In2O3 interface. Therefore, even if the E changes very little, the R of the sensor will change greatly, which significantly enhance the sensing performance of Co3O4/In2O3 heterojunction [57]. Therefore, the unique p-n heterojunction structure of Co3O4/In2O3 is greatly attributed to the super sensing performance for NO2 detection.
4 Conclusions
In summary, we reported a high-performance NO2 gas sensor based on Co3O4/In2O3 heterostructure, which was constructed by a simple hydrothermal method. The preparation of the Co3O4/In2O3 sample was confirmed by various means. The effect of the operation temperature on the sensor response was investigated, and the optimal operation temperature of 150 °C was determined. The gas-sensing results exhibits that the Co3O4/In2O3 heterostructure sensor has sub-ppm level detection ability, good selectivity and long-term stability at low temperature, which is of significance in the practical applications for NO2 detection.
References
S.A. Vanalakar, V.L. Patil, N.S. Harale, S.A. Vhanalakar, Controlled growth of ZnO nanorod arrays via wet chemical route for NO2 gas sensor applications. Sens. Actuators B 221, 1195–1201 (2015)
V. Srivastava, K. Jain, At room temperature graphene/SnO2 is better than MWCNT/SnO2 as NO2 gas sensor. Mater. Lett. 169, 28–32 (2016)
E. Oh, H.Y. Choi, S.H. Jung, S. Cho, J.C. Kim, K.H. Lee, High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuators B 141, 239–243 (2009)
S.R. Gawali, V.L. Patil, V.G. Deonikar, S.S. Patil, Ce doped NiO nanoparticles as selective NO2 gas sensor. J. Phys. Chem. Solids 114, 28–35 (2018)
L.P. Gao, Z.X. Cheng, Q. Xiang, Y. Zhang, J.Q. Xu, Porous corundum-type In2O3 nanosheets: synthesis and NO2 sensing properties. Sens. Actuators B 208, 436–443 (2015)
X.L. Hu, L.Y. Tian, H.B. Sun, B. Wang, Y. Gao, Highly enhanced NO2 sensing performances of Cu-doped In2O3 hierarchical flowers. Sens. Actuators B 221, 297–304 (2015)
Z.Y. Liu, L.M. Yu, F. Guo, S. Liu, L.J. Qi, Facial development of high performance room temperature NO2 gas sensors based on ZnO nanowalls decorated rGO nanosheets. Appl. Surf. Sci. 423, 721–727 (2017)
L.J. Qi, L.M. Yu, Z.Y. Liu, F. Guo, Y.Q. Gu, An enhanced optoelectronic NO2 gas sensors based on direct growth ZnO nanowalls in situ on porous rGO. J. Alloys Compd. 749, 244–249 (2018)
B.X. Xiao, F. Wang, C.B. Zhai, P. Wang, C.H. Xiao, Facile synthesis of In2O3 nanoparticles for sensing properties at low detection temperature. Sens. Actuators B 235, 251–257 (2016)
M.Z. Jiao, N.V. Chien, N.V. Duy, N.D. Hoa, N.V. Hieu, On-chip hydrothermal growth of ZnO nanorods at low temperature for highly selective NO2 gas sensor. Mater. Lett. 169, 231–235 (2016)
D.L. Kamble, N.S. Harale, V.L. Patil, P.S. Patil, L.D. Kadam, Characterization and NO2 gas sensing properties of spray pyrolyzed SnO2 thin films. J. Anal. Appl. Pyrolysis 127, 38–46 (2017)
K.H. Shin, S.S. Park, H.Y. Jeong, Y.W. Noh, D.J. Lee, NO2 sensing properties of bead-like TeO2 nanostructures fabricated using different O2 flow rates. Bull. Korean Chem. Soc. 36, 2688–2692 (2015)
S.X. Shi, F. Zhang, H.M. Lin, Q. Wang, E. Shi, Enhanced triethylamine-sensing properties of P-N heterojunction Co3O4/In2O3 hollow microtubes derived from metal-organic frameworks. Sens. Actuators B 262, 739–749 (2018)
J.W. Ma, H.Q. Fan, H.L. Tian, X.H. Ren, C. Wang, Ultrahigh sensitivity and selectivity chlorine gas sensing of In2O3 hollow microtubules by bio-template method with degreasing cotton. Sens. Actuators B 262, 17–25 (2018)
B.X. Xiao, D.X. Wang, S.L. Song, C.B. Zhai, F. Wang, Fabrication of mesoporous In2O3 nanospheres and their ultrasensitive NO2 sensing properties. Sens. Actuators B 248, 519–526 (2017)
X.M. Xu, P.L. Zhao, D.W. Wang, P. Sun, L. You, Preparation and gas sensing properties of hierarchical flower-like In2O3 microspheres. Sens. Actuators B 176, 405–412 (2013)
X.M. Xu, D.W. Wang, J. Liu, P. Sun, Y. Guan, Template-free synthesis of novel In2O3 nanostructures and their application to gas sensors. Sens. Actuators B 185, 32–38 (2013)
O. Bierwagen, J.S. Speck, Plasma- assisted molecular beam epitaxy of Sn-dope d In2O3: Sn incorporation, structural changes, doping limits, and compensation. Phys. Status Solidi A 211, 48–53 (2014)
H. Baqiah, N.B. Ibrahim, M.H. Abdi, S.A. Halim, Electrical transport, microstructure and optical properties of Cr-doped In2O3 thin film prepared by sol-gel method. J. Alloys Compd. 575, 198–206 (2013)
Y. Chao, L.H. bing, G.J. zhi, Z. Ying, Improved NO2 sensing properties at low temperature using reduced graphene oxide nanosheet-In2O3 heterojunction nanofibers. J. Alloys Compd. 741, 908–917 (2018)
M.D. Ding, N. Xie, C. Wang, X.Y. Kou, H. Zhang, Enhanced NO2 gas sensing properties by Ag-doped hollow urchin-like In2O3 hierarchical nanostructures. Sens. Actuators B 252, 418–427 (2017)
H. Kim, S. An, C.H. Jin, C. Lee, Structure and NO2 gas sensing properties of SnO2-core/In2O3-shell nanobelts. Curr. Appl. Phys. 12, 1125–1130 (2012)
M. Donarelli, S. Prezioso, F. Perrozzi, F. Bisti, M. Nardone, Response to NO2 and other gases of resistive chemically exfoliated MoS2-based gas sensors. Sens. Actuators B 207, 602–613 (2015)
N.D. Chinh, N.V. Toan, V.V. Quang, N.V. Duy, N.D. Hoa, Comparative NO2 gas-sensing performance of the self-heated individual, multiple and networked SnO2 nanowire sensors fabricated by a simple process. Sens. Actuators B 201, 7–12 (2014)
Y.H. Kim, S.J. Kim, Y.J. Kim, Y.S. Shim, S.Y. Kim, Self-activated transparent all-graphene gas sensor with endurance to humidity and mechanical bending. ACS Nano 9, 10453–10460 (2015)
K.K. Sadasivuni, D. Ponnamma, H.U. Ko, H.C. Kim, L.D. Zhai, Flexible NO2 sensors from renewable cellulose nanocrystals/iron oxide composites. Sens. Actuators B 233, 633–638 (2016)
Q.T. Nguyet, N.V. Duy, N.T. Phuong, N.N. Trung, C.M. Hung, Superior enhancement of NO2 gas response using n-p-n transition of carbon nanotubes/SnO2 nanowires heterojunctions. Sens. Actuators B 238, 1120–1127 (2017)
B.W. Zhang, W.Y. Fu, X.W. Meng, A. Ruan, P.Y. Su, H.B. Yang, Synthesis of actinomorphic flower-like SnO2 nanorods decorated with CuO nanoparticles and their improved isopropanol sensing properties. Appl. Surf. Sci. 456, 586–593 (2018)
V.P. Dinesh, A. Sukhananazerin, An emphatic study on role of spill-over sensitization and surface defects on NO2 gas sensor properties of ultralong ZnO@Au heterojunction NRs. J. Alloy Compds. 712, 811–821 (2017)
C.W. Zou, J. Wang, W. Xie, Synthesis and enhanced NO2 gas sensing properties of ZnO nanorods/TiO2 nanoparticles heterojunction composites. J. Colloid Interface Sci. 478, 22–28 (2016)
W.Y. Zhang, M. Hu, X. Liu, Y.L. Wei, N. Li, Synthesis of the cactus-like silicon nanowires/tungsten oxide nanowires composite for room-temperature NO2 gas sensor. J. Alloy Compds. 679, 391–399 (2016)
N. Chen, X.G. Li, X.Y. Wang, J. Yu, J. Wang, Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens. Actuators B 188, 902–908 (2013)
B. Zhang, M. Cheng, G.N. Liu, Y. Gao, L.J. Zhao, Room temperature NO2 gas sensor based on porous Co3O4 slices/reduced graphene oxide hybrid. Sens. Actuators B 263, 387–399 (2018)
T.T. Zhou, T. Zhang, J.N. Deng, R. Zhang, Z. Lou, P-type Co3O4 nanomaterials-based gas sensor: Preparation and acetone sensing performance. Sens. Actuators B 242, 369–377 (2017)
K. Song, X.Q. Meng, J.L. Zhang, Y. Zhang, A simple grinding-calcination approach to prepare the Co3O4-In2O3 heterojunction structure with high-performance gas-sensing property toward ethanol. RSC Adv. 6, 105262–105269 (2016)
M. Ali, P. Sunghoon, K. Hyejoon, S.G. Joo, Acetone sensors based on In2O3-Co3O4 composite nanoparticles. J. Nanosci. Nanotechnol. 17, 4087–4090 (2017)
Q.Y. Yang, X.B. Cui, J.Y. Liu, J. Zhao, Y.L. Wang, A low temperature operating gas sensor with high response to NO2 based on ordered mesoporous Ni-doped In2O3. RSC Adv. 40, 2376–2382 (2016)
Z.Y. Zhang, L.P. Zhu, Z. Wen, Z.Z. Ye, Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sens. Actuators B 238, 1052–1059 (2017)
D. Zhang, Y. Cao, P. Li, J. Wu, X. Zong, Humidity-sensing performance of layer-by-layer self-assembled tungsten disulfide/tin dioxide nanocomposite. Sens. Actuators B 265, 529–538 (2018)
R. Dong, L.P. Zhang, Z.Y. Zhu, J.D. Yang, X.L. Gao, Fabrication and formaldehyde sensing performance of Fe-doped In2O3 hollow microspheres via a one-pot method. CrystEngComm. 19, 562–569 (2017)
L.P. Huo, X. Yang, Z.W. Liu, X. Tian, T.J. Qi, Modulation of potential barrier heights in Co3O4/SnO2 heterojunctions for highly H2-selective sensors. Sens. Actuators B 244, 694–700 (2017)
B.F. Wu, L.L. Wang, H.Y. Wu, K. Kan, G. Zhang, Templated synthesis of 3D hierarchical porous Co3O4 materials and their NH3 sensor at room temperature. Microporous Mesoporous Mater. 225, 154–163 (2016)
D. Zhang, Z. Wu, X. Zong, Y. Zhang, Fabrication of polypyrrole/Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sens. Actuators B 274, 575–586 (2018)
C.W. Na, J.H. Kim, H.J. Kim, H.S. W, A. Gupta, Highly selective and sensitive detection of NO2 using rGO-In2O3 structure on flexible substrate at low temperature. Sens. Actuators B 255, 1671–1679 (2018)
F.B. Gu, R. Nie, D.M. Han, Z.H. Wang, In2O3-graphene nanocomposite based gas sensor for selective detection of NO2 at room temperature. Sens. Actuators B 219, 94–99 (2015)
Z.X. Cheng, L.Y. Song, X.H. Ren, Q. Zheng, J.Q. Xu, Novel lotus root slice-like self-assembled In2O3 microspheres: synthesis and NO2-sensing properties. Sens. Actuators B 176, 258–263 (2013)
W. Yang, P. Wan, X.D. Zhou, J.M. Hu, Additive-free synthesis of In2O3 cubes embedded into graphene sheets and their enhanced NO2 sensing performance at room temperature. ACS Appl. Mater. Interfaces 6, 21093–21100 (2014)
H. Kim, S. An, C. Jin, C.M. Lee, Structure and NO2 gas sensing properties of SnO2-core/In2O3-shell nanobelts. Curr. Appl. Phys. 12, 1125–1130 (2012)
Y.J. Kwon, H.G. Na, S.Y. Kang, M.S. Choi, Attachment of Co3O4 layer to SnO2 nanowires for enhanced gas sensing properties. Sens. Actuators B 239, 180–192 (2017)
D.G. Ding, Q.Z. Xue, W.B. Lu, Y. Xiong, J.Q. Zhang, Chemically functionalized 3D reticular graphene oxide frameworks decorated with MOF-derived Co3O4: towards highly sensitive and selective detection to acetone. Sens. Actuators B 259, 289–298 (2018)
N.M. Shaalan, M. Rashad, A.H. Moharram, M.A. Abdel-Rahim, Promising methane gas sensor synthesized by microwave-assisted Co3O4 nanoparticles. Mater. Sci. Semicond. Process. 46, 1–5 (2016)
L.D. Xu, X.C. Zhang, P.C. Wang, D.C. Guo, Design and synthesis of p-n conversion indium-oxide-based gas sensor with high sensitivity to NOx at room-temperature. Chem. Sel. 3, 2298–2305 (2018)
N.K. Pawar, Gas sensing characteristics of pure and ZnO-modified Fe2O3 thick films. Lect. Notes Electr. Eng. 83, 123–132 (2011)
Y.F. Wang, F.D. Qu, J. Liu, Y. Wang, J.R. Zhou, S.P. Ruan, Enhanced H2S sensing characteristics of CuO-NiO core-shell microspheres sensors. Sens. Actuators B 209, 515–523 (2015)
D. Zhang, C. Jiang, P. Li, Y. Sun, Layer-by-Layer self-assembly of Co3O4 nanorod-decorated MoS2 nanosheet-based nanocomposite toward high-performance ammonia detection. ACS Appl. Mater. Interfaces 9, 6462–6471 (2017)
C.L. Zhu, H.L. Yu, Y. Zhang, T.S. Wang, Q.Y. Ouyang, L.H. Qi, Y.J. Chen, X.Y. Xue, Fe3O4/TiO2 tube-like nanostructures: synthesis, structural transformation and the enhanced sensing properties. ACS Appl. Mater. Interfaces 4, 665–671 (2012)
C. Liu, L. Zhao, B. Wang, P. Sun, Q. Wang, Y. Gao, X. Liang, T. Zhang, G. Lu, Acetone gas sensor based on NiO/ZnO hollow spheres: fast response and recovery, and low (ppb) detection limit. J. Colloid Interface Sci. 495, 207–215 (2017)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51777215), the Key Research & Development Plan Project of Shandong Province (2018GSF117002), the Fundamental Research Funds for the Central Universities of China (18CX07010A), the Open Fund of Key Laboratory of Marine Spill Oil Identification and Damage Assessment Technology, State Oceanic Administration of China (No. 201801).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Wu, D., Cao, Y. et al. Construction of Co3O4 nanorods/In2O3 nanocubes heterojunctions for efficient sensing of NO2 gas at low temperature. J Mater Sci: Mater Electron 29, 19558–19566 (2018). https://doi.org/10.1007/s10854-018-0087-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0087-9