Abstract
The electromigration (EM) degrades the structural, mechanical and electrical properties of solder joints. An effort has been made to investigate the effects of the Ni nanoparticles (NP) doped flux on the mechanical properties and electrical resistance of SAC305 solder joints subjected to EM. SAC305 solder joints were prepared using NP-doped flux. A current density of 3 × 103 A/cm2 was applied to the joints at a constant temperature of 150 °C. Tensile tests were performed before and after the EM tests. Results reveal that after the addition of Ni NP-doped flux, the mechanical strength improved before and after EM. After EM, the fracture path for doped solder joint did not migrate to the cathode side as compared to un-doped solder. Ni NP also improved the electrical resistance and lifetime of the solder joint. The use of Ni NP-doped flux thus minimized the effects of EM and improved the mechanical and electrical performance of the solder joints.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Increased miniaturization of electronic devices requires downscaling the size of interconnects and consequently increases the input current density in solder joints [1]. The increase in current density significantly increases the occurrence of electromigration (EM) failures [2]. During EM, electrons under high current density apply force on atoms present on the cathode interface [3, 4]. As Cu atoms require low activation energy compared to Sn atoms, the Cu atoms migrate rapidly during the EM [5]. Migration of Cu atoms from the cathode to anode side is known to be one of the main reasons of EM failures [6]. Due to rapid migration of Cu atoms from the cathode towards the anode, voids form at the cathode side of the joint. It has been found that voids created by EM weaken the cathode interface. This results in the transfer of the fracture path from the bulk to the cathode side [7]. The formation of voids during EM reduces the strength of solder joints and also changes the fracture mode from ductile to brittle [8].
In order to reduce EM damage, it is important to retard Cu diffusion from the initiation points. A technique involving the use of nanoparticle (NP) doped flux was developed in the recent years to modify the interfaces of solder joints [9]. It was found that the use of Ni NP-doped flux at solder substrate interfaces resulted in the incorporation of Ni atoms in the solder joint during reflow through reactive dissolution. The incorporation of Ni atoms made the interfacial intermetallic compound (IMC) layer more stable during EM. As a result, Cu diffusion was reduced and EM damages like void and crack formation at the cathode interface were suppressed [7]. The effects of Ni NP on the mechanical and electrical performance of SAC solder joints under EM have not been investigated in the past. In this study, an effort has been made to investigate the effects of Ni NP addition through flux doping on the mechanical strength and electrical resistance of Sn-based solder joints subjected to electromigration.
2 Experimental
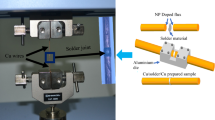
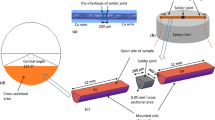
2 wt% Ni nanoparticles (Accumet Materials, Co., USA) were mixed with a water-soluble flux (Sparkle Flux WF-6317, Japan) to prepare the nanoparticle doped flux. The average size of NP was 44 nm. Further details on sample preparation are given in [7]. 2 wt% of Ni-NP were mechanically mixed with water-soluble flux for 30 min. Two copper wires with the diameter of 0.5 mm were used. The length of the copper wires was 50 mm. One end of each copper wire was cleaned and polished. After polishing, the polished ends of copper wires were carefully dipped into the flux so that most of the flux remains at the end. The fluxed wires were then placed in an aluminum die. Line type solder joints, Cu/Solder/Cu, were prepared as illustrated in the Fig. 1. Ni-doped flux was applied on both interfaces of the solder joint prior to the reflow which was conducted at 245 °C for 45 s. The solder joints were then subjected to EM test. A current density of 5 × 103 A/cm2 was applied at a constant temperature of 150 °C for a time period of 192 h. After the EM tests, tensile tests were conducted on the joints in a Shimadzu AGS-X Universal tensile testing machine using a 50 N load cell. The tensile tests were also done on reflowed samples for comparison purposes. The tests were repeated 5 times for each condition at a strain rate of 6.59 × 10−5/s. The stress–strain curves and tensile strength were recorded during the tensile tests. Another set of SAC305 and Ni-doped SAC305 samples was prepared to measure the electrical resistance during EM test. The constant current density of 5 × 103 A/cm2 was applied at a maintained temperature of 150 °C. The experiment was conducted in silicone oil bath. A pure silicone oil was used to maintain the temperature of the sample. The maximum time duration for this set of tests was 700 h. The EM temperature and current density were same as described above. The resistance was measured by a multimeter (FLUKE 45 dual display multimeter) during the EM test. The tests were repeated 3 times under the same condition.
3 Results
Figure 2a, b present the stress–strain curves for Ni NP-doped flux solder before and after EM tests. The solder joints possess significantly higher values of maximum stress before EM tests (Fig. 2a). EM causes a lowering of the maximum tensile stress values (Fig. 2b). No significant changes are found in the values of maximum strain% before and after EM tests (Fig. 2a, b). Table 1 illustrates the average tensile strength of Ni NP-doped solder joints calculated from the Fig. 2a, b. Corresponding values collected from the literature are also shown for comparison purposes. In the table, the average strength of Ni-doped solder is found to be 100.99 MPa before EM. After EM, the average strength went down to 77.23 MPa. In our previous study, the tensile strength for undoped solder was found to be 60.34 MPa before EM. After EM, the strength of the undoped solder joints abruptly dropped to 22.36 MPa (Table 1). It is thus seen that Ni NP doping increased the joint strength from 60.34 to 100.99 MPa before EM. Ni NP doping assisted in maintaining the joint strength at 77.23 MPa after 192 h of EM, while undoped solder possessed only a fraction of the strength, 22.36 MPa after the same EM period. A comparison of our previous joint strength values [10] for undoped solder joints with available literature values [8, 11, 12] can also be seen in Table 1. Although values of different parameters used in the investigation described in the literature are not exactly the same as ours, our tensile strength values of undoped joints are reasonably close to literature values before and after EM [10]. In the past, the strength of Co-doped solder was also investigated before and after the EM test. It is found that Co-doped solder showed similar behavior as Ni-doped solder, that is Co-NP doping helped to maintain high tensile strength after EM. (Table 1) [10].
Table 2 shows the average strain % of Ni NP containing solder calculated from Fig. 2a and b. Literature values are also shown for comparison. The average strain % of Ni-doped solder was 0.37% before the EM test and 0.35% after the EM test. Although the average strain value of Ni NP-doped joints after EM seem higher, In our previous study, the average value of maximum strain % of undoped solder joints was found to be 0.65 and 0.29% respectively before and after EM [10]. Literature values of strain of undoped SAC solder joints [8, 12] vary from each other (Table 2). But these are generally higher than the corresponding values found in our earlier work [10] for undoped solder. Strain values of Co-NP doped solder reported in the past [10] are close to that of Ni NP-doped solder in the present case (Table 2).
Figure 2c, d present photographs of typical joints after fracture in tensile tests. Ni-doped solder joints showed a ductile fracture, the fracture path is within the solder bulk both before and after EM test. In the case of un-doped solder, as reported in the literature [8, 10, 12, 13], the fracture mode changed from ductile to brittle type after EM test, and the fracture path was shifted from the bulk to the cathode interfaces [8, 10, 12, 13]. The Ni NP doping helped in maintaining the ductile bulk fracture even after EM. Similar behavior was also observed for Co-NP doped samples [10]. Figure 3a, b describe the electrical resistance of un-doped and Ni-doped solder joint as a function of EM time. In un-doped solder joints, the electrical resistance tends to increase with EM time. The samples averagely failed before 500 h as indicated by the abrupt rise in the resistance values. In the case of Ni-doped solder, the electrical resistance slightly increased but the sample did not fail even upto700 h of EM test.
4 Discussion
It was found in our earlier work [7] that when Ni NP-doped flux was used in the solder joint preparation, the nanoparticles underwent reactive dissolution during reflow and Ni entered into the solder joint. Ni then acted as a minor alloy addition. In the present study, the overall Ni content in the solder was estimated based on the amount of Ni-doped flux at the interface between solder ball/copper wire, amount of Ni NP in the flux and the rate of incorporation on Ni NP into solder as determined in our previous study [14, 15]. Estimation showed that about 500 ppm of Ni was incorporated into solder from each interface. So the total amount of Ni in the solder was estimated to be 1000 ppm which entered from both interfaces.
The distribution of Ni NP at interfaces was investigated by EDX in the past [10]. It was found that Ni was concentrated in the interfacial Cu6Sn5 layer and also in the IMC particles in the matrix. The Ni content at the interfacial IMC was 2.26% near to the solder and was 0.96% at a place close to Cu substrate [7].
Ni and Cu have similar face-centered cubic crystallographic structure. This is why Ni atoms have a better solubility in Cu6Sn5 [7]. It has been found that Ni atoms substitute the Cu atoms in the sublattice of Cu6Sn5 IMC joint at interfaces and in the bulk of the solder joint [16]. The substitution of Cu with Ni reduce the formation energy of Cu6Sn5 and change the IMC composition from Cu6Sn5 to (Cu, Ni)6Sn5. The formation enthalpies of Cu6Sn5 to (Cu, Ni)6Sn5 were calculated by Zhang et al. [17]. It was found that Ni reduces the formation enthalpies of Cu6Sn5 phase. The reduction in formation enthalpies was found to be in the range of − 40 to − 100 meV/atom in Ni-containing Cu6Sn5 IMC phases [17]. Gao et al. have also shown using a thermodynamic calculation that (Cu, Ni)6Sn5IMCs is comparatively more stable than Cu6Sn5 [18]. They concluded that Ni and Sn have a higher affinity as compared with Cu and Sn. The higher affinity of Ni and Sn increases the nucleation rate of IMC in interfacial layer [18]. This also promotes a planar interfacial IMC morphology [7]. In a previous study [16], after addition of Ni NPs, the interfacial IMC morphology was refined and was more planar which indicates that the nucleation rate of IMC was promoted after addition of Ni NPs. The addition of Ni also reduces and refines the grain size of IMC which improves the mechanical properties of the solder [18, 19]. Moreover, Ni addition also suppresses the growth of Cu3Sn and reduces void formation [20] which also improve the mechanical properties of the solder joint.
During EM, the heavy current wind applies force on the atoms present at cathode interfaces of the solder joint. It was found that Cu atoms require low activation energy compared to Sn atoms which result in rapid migration of Cu atoms from the cathode to anode side during EM [5]. During EM, Cu migration for the cathode to the anode is expected to occur in two ways: one is Cu migration from Cu substrate and another one is Cu migration from interfacial IMC layer at the cathode side. In the first case, the Cu atoms from the Cu substrate migrate through the interfacial IMC layer during the EM. In scalloped shape interfacial IMC layers, the Cu atoms mostly migrate through the valley areas [21]. Further explanation is given in Fig. 3c. As the Ni atoms change the morphology of interfacial IMC from scallop to planar shape so it eliminates this easy Cu diffusion path [21] (Fig. 3d). When diffused Cu atoms try to migrate from the Cu substrate to anode side through the planar shape (Cu, Ni)6Sn5 interfacial IMC layer then Ni atoms quickly react with diffused Cu atoms and reduce further diffusion [7]. In the second type of Cu migration, Cu atoms rapidly migrate from Cu6Sn5 interfacial IMC layer in undoped solder and reduce the thickness of IMC layer significantly. But when doped with Ni atoms they reduce the Cu diffusion significantly from the (Cu, Ni)6Sn5 interfacial IMC layer due to its higher thermodynamic stability [18]. This retards the rapid reduction of IMC thickness at the cathode side. Ni controls the rapid growth of IMC at anode interfaces and reduces the void and crack formation at the cathode side.
The structural degradation of the interfaces can impose serious effects on mechanical performance of the solder joint. In an earlier study [10] the effect of EM on SAC305 solder joint was investigated. It was observed that due to EM, cracks, and damage were formed at the cathode interface of the solder joint. These cracks and damage weakened the cathode interface and the fracture migrated to cathode interfaces [10]. The fracture was cup and cone shaped with ductile behavior before EM, but after 192 h it converted from ductile to brittle [10]. Ren et al. [8] found that EM converts the ductile to brittle fracture and fracture path migrated to the cathode interfaces of SAC solder joint. The value of maximum strain and stress also reduced [8]. Kumar et al. also concluded that EM caused the brittle fracture in Sn-Ag solder due to high current density and as a result, the also degraded [13]. Wang et al. reported that by increasing the current stressing time the shear strength of SAC solder decreased and the fracture migrated to the cathode interfaces with brittle behavior [22]. Similar results were also reported elsewhere [12]. In our previous study, it was found that after the addition of Ni NP, the formation of cracks and damage were significantly reduced during the EM test [7, 23]. Hence, reduction of cracks and damage can reduce the degradation of the strength of the solder joint during EM. In the present study, it is observed that addition of Ni NP significantly reduced the degradation of solder strength during EM (Fig. 2b). No significant effect of EM was found on the fracture behavior of Ni NP containing solder joint. The maximum stress slightly decreased but no significant effect found for maximum strain values after EM test. The addition of Ni NP-doped flux upgraded the structural properties of SAC305 solder joint which imposed positive effects of mechanical strength. Therefore, by correlating of our findings with literature, it can be concluded that the thermodynamic stability of IMC, less variation in IMC thickness, reduction in EM damages, favourable morphology of interfacial IMC and reduction in Cu diffusion could be some of the possible reasons for improvement in electrical and mechanical properties of Ni NP-doped flux containing solder joint after the EM.
5 Conclusion
This report reveals that after the addition of Ni NP through flux doping improved the mechanical strength of reflowed samples and reduced the degradation of mechanical strength after EM test. The fracture path did not shift towards the cathode and stayed inside the solder bulk even after EM test. Ni NP-doped flux solder also showed better performance in electrical resistance test and the sample did not fail until the end of the test conducted for 700 h.
References
L. Ma, G. Xu, J. Sun, F. Guo, X. Wang, Effects of Co additions on electromigration behaviors in Sn–3.0 Ag–0.5 Cu-based solder joint. J. Mater. Sci. 46(14), 4896–4905 (2011)
H. Ji, M. Li, S. Ma, M. Li, Ni 3 Sn 4-composed die bonded interface rapidly formed by ultrasonic-assisted soldering of Sn/Ni solder paste for high-temperature power device packaging. Mater. Des. 108, 590–596 (2016)
M. Li, H. Chang, X. Pang, L. Wang, Y. Fu, Abnormal accumulation of intermetallic compound at cathode in a SnAg3. 0Cu0. 5 lap joint during electromigration. J. Phys. D 44(11), 115501 (2011)
X. Zhang, H. Liu, J. Guo, J. Shang, Inhibition of electromigration in eutectic SnBi solder interconnect by plastic prestraining. J. Mater. Sci. Technol. 27(11), 1072–1076 (2011)
T. Yang, J. Yu, C. Li, Y. Lin, C. Kao, Dominant effects of Sn orientation on serrated cathode dissolution and resulting failure in actual solder joints under electromigration. J. Alloys Compd. 627, 281–286 (2015)
C. Wang, K. Li, Study of electromigration resistance of (Pd, Ni) Sn 4 phase in lead-free solder joints. J. Alloys Compd. 654, 546–553 (2016)
M.N. Bashir, A. Haseeb, A.Z.M.S. Rahman, M. Fazal, C. Kao, Reduction of electromigration damage in SAC305 solder joints by adding Ni nanoparticles through flux doping. J. Mater. Sci. 50(20), 6748–6756 (2015)
F. Ren, J.-W. Nah, K. Tu, B. Xiong, L. Xu, J.H. Pang, Electromigration induced ductile-to-brittle transition in lead-free solder joints. Appl. Phys. Lett. 89(14), 141914 (2006)
G. Sujan, A. Haseeb, A. Afifi, Effects of metallic nanoparticle doped flux on the interfacial intermetallic compounds between lead-free solder ball and copper substrate. Mater. Charact. 97, 199–209 (2014)
M.N. Bashir, A.S.M.A. Haseeb, A.Z.M.S. Rahman, M.A. Fazal, Effect of cobalt doping on the microstructure and tensile properties of lead free solder subject to electromigration. J. Mater. Sci. Technol. 32, 1129–1136 (2016)
G. Wei, J. Yao, Y. Shi, The influence of electromigration and aging on the reliability of SnAgCu lead-free solder joint at 100 °C, in 12th international conference on electronic packaging technology and high density packaging (ICEPT-HDP), IEEE, 2011, pp. 1–5
L. Zhang, Z. Wang, J. Shang, Current-induced weakening of Sn3. 5Ag0.7Cu Pb-free solder joints. Scr. Mater. 56(5), 381–384 (2007)
A. Kumar, Y. Yang, C.C. Wong, V. Kripesh, Z. Chen, Effect of electromigration on the mechanical performance of Sn-3.5 Ag solder joints with Ni and Ni-P metallizations. J. Electron. Mater. 38(1), 78–87 (2009)
G. Sujan, A. Haseeb, H. Nishikawa, M. Amalina, Interfacial reaction, ball shear strength and fracture surface analysis of lead-free solder joints prepared using cobalt nanoparticle doped flux. J. Alloys Compd. 695, 981–990 (2017)
A. Haseeb, M. Arafat, S. Tay, Y. Leong, Effects of metallic nanoparticles on interfacial intermetallic compounds in Tin-based solders for microelectronic packaging. J. Electron. Mater. 46(10), 5503–5518 (2017)
S. Tay, A. Haseeb, M.R. Johan, P. Munroe, M. Quadir, Influence of Ni nanoparticle on the morphology and growth of interfacial intermetallic compounds between Sn–3.8 Ag–0.7 Cu lead-free solder and copper substrate. Intermetallics 33, 8–15 (2013)
Y. Zhang, D.-W. Yuan, J.-H. Chen, G. Zeng, T.-W. Fan, Z.-R. Liu, C.-L. Wu, L.-H. Liu, Alloying effects on the phase stability and mechanical properties of doped Cu-Sn IMCs: a first-principle study. J. Electron. Mater. 45, 4018–4027 (2016)
F. Gao, T. Takemoto, H. Nishikawa, Effects of Co and Ni addition on reactive diffusion between Sn–3.5 Ag solder and Cu during soldering and annealing. Mater. Sci. Eng. A 420(1), 39–46 (2006)
T. Laurila, V. Vuorinen, M. Paulasto-Kröckel, Impurity and alloying effects on interfacial reaction layers in Pb-free soldering. Mater. Sci. Eng. R 68(1), 1–38 (2010)
T. Maeshima, H. Ikehata, K. Terui, Y. Sakamoto, Effect of Ni to the Cu substrate on the interfacial reaction with Sn-Cu solder. Mater. Des. 103, 106–113 (2016)
R. Zhao, L. Ma, Y. Zuo, S. Liu, F. Guo, Retarding electromigration in lead-free solder joints by alloying and composite approaches. J. Electron. Mater. 42(2), 280–287 (2013)
X. Wang, Q. Zeng, Q. Zhu, Z. Wang, J. Shang, Effects of current stressing on shear properties of Sn-3.8 Ag-0.7 Cu solder joints. J. Mater. Sci. Technol. 26(8), 737–742 (2010)
M.N. Bashir, A. Haseeb, S. Rahman, A. Zayed, M. Fazal, Effect of Ni nanoparticles on intermetallic compounds formation in SAC305 solder joint under high current density, in 2014 IEEE 36th international electronics manufacturing technology conference (IEMT), IEEE, 2014, pp. 1–5
Acknowledgements
This study was financially supported by High Impact Research Grant (UM.C/HIR/MOHE/ENG/26, Grant No. D000026-16001) provided by Malaysian Ministry of Higher Education and postgraduate research grant University of Malaya Project Number PG146-2016A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bashir, M.N., Haseeb, A.S.M.A. Improving mechanical and electrical properties of Cu/SAC305/Cu solder joints under electromigration by using Ni nanoparticles doped flux. J Mater Sci: Mater Electron 29, 3182–3188 (2018). https://doi.org/10.1007/s10854-017-8252-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8252-0