Abstract
For the first time, the hierarchical structures of Li0.35Zn0.3Fe2.35O4(LZFO)/polyaniline nanocomposites were successfully synthesized by interfacial polymerization. Firstly, the LZFO particles were prepared by the sol–gel method, and subsequently the PANI nanorods, composed of nanoneedle-like PANI, were grafted on the surface of the LZFO. A novel microtopography, urchin-like, of LZFO/PANI was prepared by a simple, efficient and controllable two-step method. The crystal structure, chemical bonding states and morphology of samples were characterized by means of Fourier transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, Scanning and Transmission electron microscopy (SEM/TEM). The bandwidth of reflection loss exceeds 10 dB in the frequency was 5.56 GHz (3.36–8.48, 10.32–10.76 GHz), and the maximum reflection loss can reach − 49.4 dB at 4.96 GHz with the thickness of 5.1 mm. The enhanced microwave absorption properties of LZFO/PANI nanocomposites are mainly ascribed to the multi-level structure and the improved impedance matching, and make it a potential candidate for microwave absorption materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recent years have witnessed that the electromagnetic interference (EMI) problem becomes more and more serious [1, 2], because of the number of electronic products increasing. The cell phones, interphone, computers and other communication equipment have become an indispensable part of our modern life. These equipment cause EMI problem and should be addressed properly for their safe use [2]. Highly efficient microwave absorbing materials have gain a considerable interest for current research in this field. Hierarchical structures with complicated geometrical morphologies can provide excellent microwave absorption properties [3]. However, ferrites are widely used as good microwave absorbers due to their high saturation magnetization, great coercivity and adjustable anisotropy through ion doping [4]. Modification of ferrites with conductive polyaniline is one of the most effective track to overcome drawbacks in ferrite based microwave absorbers [5].
In this paper, the hierarchical structures of LZFO/PANI nanocomposites have been prepared by a multi-step route and the microwave absorption properties were analyzed in detail. The results of microwave absorption properties show that such novel composite is an attractive candidate for microwave absorbing materials.
2 Experimental
LZFO/PANI-nanorod composites were prepared from the organic phase and an aqueous phase by the interfacial reaction. Nanocrystalline Li0.35Zn0.3Fe2.35O4 particles (LZFO) were synthesized from a deep green wet gel through conventional sol–gel autoignition process. Shortly after, LiNO3 (7 mmol), Zn(NO3)2·6H2O (6 mmol), Fe(NO3)3·9H2O (47 mmol) and citric acid (65 mmol) were added to the deionized water (70 ml) at the room temperature to make a clean solution under constant magnetic stirring. Nanocrystalline Li0.35Zn0.3Fe2.35O4 particles can be obtained by calcinating grinded xerogel loose powder that was prepared from wet gels. The citric acid was used as a complexing agent to form wet gels. Then, 0.03 g of sodium dodecyl benzene sulfonate (SDBS) and 1.5 g of ferrites were suspended in 150 ml of ethanol solution and treated by mechanical agitation for 2 h. The reaction, between LZFO and SDBS, that takes place between the structural hydroxyl on the ferrite and sulfite on SDBS, which led to the generation of surface-modified LZFO [6, 7]. Subsequently, 5 mmol aniline was dissolved in 50 ml carbon tetrachloride to form the organic phase (Solution A). 1.14 g of ammonium persulfate, 4.14 ml of hydrochloric acid, and 1.0 g of SDBS-LZFO were added to 50 ml deionized water to form the aqueous phase (solution B). The solution B was added to solution A drop by drop to form a clear water-organic boundary. Deep green LZFO/PANI-nanorod powder was acquired after filtering washing and drying .
The obtained products were characterized by Fourier transform infrared spectrophotometer (Bruker Vector 33), XRD (Cu Kα radiation of wavelength λ = 1.5406 Ǻ, BRUKER), X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi), field emission scanning electron microscopy (FE-SEM, Hitachi S-4800N), transmission electron microscopic (TEM, Tecnai 12). The electromagnetic parameter of deep green power was measured by Agilent PNA N5224A vector network analyzer using the coaxial-line method in the frequency range of 2–18 GHz.
3 Results and discussion
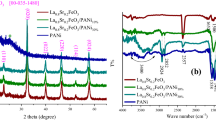
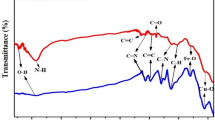
Figure 1 shows the FTIR (a), XRD (b) and XPS (c) of PANI, LZFO/PANI composite, and LZFO respectively. The characteristic bands of PANI that are attributed to C=C stretching vibration of quinoid rings and C=N stretching vibration of benzenoid rings respectively can be observed at about 1567 and 1479 cm−1 in the FTIR spectra of LZFO/PANI composite. The peaks at approximately 1300 and 1244 cm−1 are assigned to the N–H bending and C–N stretching of secondary aromatic amine respectively. In addition, the peak at 1118 cm−1 is a vibrational mode of N=O=N. The peak at about 802 cm−1 represents an out-plane bending vibration of the C–H bond. The FTIR spectrum of LZFO/PANI shows two strong characteristic absorption peaks near 3432 and 588 cm−1, which are attributed to structural hydroxyl (–OH) stretching vibration and the M–O bond (M: Li, Zn, and Fe) respectively [8]. All the characteristic peaks of PANI and LZFO can be found in the LZFO/PANI FTIR spectrum. In addition, all peaks in LZFO/PANI composites are red-shifted slightly, which indicates that the chemical reaction occurred between the PANI and LZFO closely matching the previous reports [5]. The XRD patterns of the PANI, LZFO/PANI and LZFO are shown in Fig. 1. The observed peaks, which are located at 2θ of 30°, 35.5°, 37.2°, 43.2°, 53.6°, 57.1°, 62.7°, and 74.2°, are indexed to the (220), (311), (222), (400), (442), (511), (440), and (533) [9] crystal plane reflections of the spinel structure (space group Fd3m), respectively [3]. The characteristic peaks for LZFO in the XRD pattern of LZFO and LZFO/PANI composite have been matched with the standard XRD pattern (JCPDS No. 71-1264). Besides, a weak broad peak at 2θ of 18° in the XRD of LZFO/PANI is attributed to the polymer chains of PANI according to previously reported work [8], which confirms the above mentioned the FTIR results. In Fig. 3c it can be seen that C 1s, O 1s, N 1s, Zn 2p3, Fe 2p, and Li 1s were recorded. The C 1s, O 1s, N 1s, Zn 2p3, Fe 2p, and Li 1s show peaks in the range of 280–293, 526–538, 394–410, 1016–1029, 704–739, and 50–64 eV, respectively. The results of calculations of the percentage of the atomics are listed in the table inserted in Fig. 3c. The final value of the weight ratio of LZFO/PANI was about 1.024 calculated by the percentage of the atomics.
Figure 2b shows a typical SEM of LZFO ferrite ,which presents that the grain size of cubic-like ferrite particles was about 1.8 μm. From SEM image labeled (a) of LZFO/PANI nanocomposites, PANI nanorods those the average diameter of which is about 0.61 μm and PANI nano-particles those the average grain size is about 0.55 μm were obtained. Those are ferrites which were highlighted by a red wireframe. Polymers often appear light white under high voltage in SEM photos, while ferrites appear deep dark color. Besides, the size of the red wireframe was equal to that of 3–4 ferrites gathered, the ferrite grain is easy to reunite because of magnetism and surface tension. It is evident from Fig. 2d that the PANI nanorods and nano-particles were composed of nano-sized needle-like PANI with the average diameter of which was about 11.64 nm. Figure 2c, d clearly show the formation of multi-level structure of LZFO/PANI composites. Nanoneedle polyaniline gathered to form polyaniline fibers, and then the polyaniline fibers were grafted on the surface of LZFO so that urchin-like LZFO/PANI nanocomposites were prepared. The multi-level structure of LZFO/PANI composites improved the performance of absorbing wave significantly. At the time of the electromagnetic wave reach the surface of LZFO/PANI nanocomposites, conductive polyaniline is assumed to act as the transmission channel to transmit electromagnetic waves to ferrite, which improved the matched characteristic impedance [8].
The reflection loss (RL,dB) of the electromagnetic radiation under normal wave incidence as the surface of a single-layer material backed by a perfect conductor could be given based on the transmission line theory [3]:
where Zin is the input impedance of the absorber, and the εr and μr are the measured relative complex permittivity and permeability, respectively. The thickness of the absorbing layer is d, c is the velocity of EM waves in free space is c and the microwave frequency is f. As seen in Fig. 3a, c, the optimal absorption peak of PANI and LZFO reach − 4.6 and − 25 dB, respectively. It can be observed in Fig. 3d that the minimum RL value of LZFO/PANI is about − 49.4 dB at 4.96 GHz with the thickness of 5.1 mm, which is greater than − 18.1 dB at 6.48 GHz of LZFO, and the corresponding adsorption frequency bandwidth (RL < − 10 dB) can reach 5.56 GHz (3.36–8.48, 10.32–10.76 GHz). Comparing with PANI and LZFO, the LZFO/PANI exhibits a breakthrough in the improvement of microwave absorption. It is worth noting that the adsorption peaks have a trend to shift toward the low frequency areas as the thickness of the material increases from 0.5 to 6.0 mm with the simultaneous decrease of minimal RL value decreases simultaneously [10]. All the above results apparently indicate that the LZFO/PANI composites exhibit a better adsorption performance and a broader bandwidth with a thinner thickness than LZFO, PANI and other microwave absorption composites, such as rGO/Fe3O4 composites (− 26.4 dB at 5.3 GHz with a thickness of 5 mm) [11], CoFe2O4 hollow sphere/graphene composites (− 18.5 dB at a thickness of 2.0 mm) [12]. The improvement in microwave absorption of the urchin-like LZFO/PANI nanocomposites can be explained as follows: Firstly, the multi-level structure of LZFO/PANI nanocomposites can contribute to the absorption of magnetic loss and dielectric loss, which is greatly good to widen frequency range of absorption and enhance reflection loss [8]. Secondly, the multi-level structure of LZFO/PANI nanocomposites with the larger aspect ratio leads to the microwave penetration more easily and less direct reflection on the surface of the composites [13]. Therefore, the LZFO/PANI nanocomposites can effectively serve as a lightweight potential microwave absorbing material in the frequency range from 2 to 18 GHz.
4 Conclusions
In conclusion, PANI nanorods composed of nanoneedle PANI, were successfully grafted on the surface of the LZFO particles and the value of the weight ratio of LZFO/PANI was about 1.024. The PANI nanorods and nano-particles were composed of nano-sized needle-like PANI, the average diameter of which was about 11.64 nm. The bandwidth of reflection loss exceeds 10 dB in the frequency was 5.56 GHz (3.36–8.48, 10.32–10.76), and the maximum reflection can loss reach − 49.4 dB at 4.96 GHz with the thickness of 5.1 mm.
References
W. Wang, S.P. Gumfekar, Q. Jiao, B. Zhao, Ferrite-grafted polyaniline nanofibers as electromagnetic shielding materials. J. Mater. Chem. C 1, 2851–2859 (2013)
H. Farrokhi, O. Khani, F. Nemati, M. Jazirehpour, Synthesis and investigation of microwave characteristics of polypyrrole nanostructures prepared via self-reactive flower-like MnO2 template. Synth. Met. 215, 142–149 (2016)
X. Zhang, Y. Huang, X. Chen, C. Li, J. Chen, Hierarchical structures of graphene@CoFe2O4@SiO2@TiO2 nanosheets: Synthesis and excellent microwave absorption properties. Mater. Lett. 158, 380–383 (2015)
G. Mu, N. Chen, X. Pan, H. Shen, M. Gu, Preparation and microwave absorption properties of barium ferrite nanorods. Mater. Lett. 62, 840–842 (2008)
M. Du, Z. Yao, J. Zhou, P. Liu, T. Yao, R. Yao, Design of efficient microwave absorbers based on multi-layered polyaniline nanofibers and polyaniline nanofibers/Li0.35Zn0.3Fe2.35O4 nanocomposite. Synth. Met. 223, 49–57 (2017)
N.N. Ab Kadir, M. Shahadat, S. Ismail, Formulation study for softening of hard water using surfactant modified bentonite adsorbent coating. Appl. Clay Sci. 137, 168–175 (2017)
W. Wang, Y. Song, Q. Liu, K. Yang, Facile synthesis and catalytic properties of silver colloidal nanoparticles stabilized by SDBS. Bull. Mater. Sci. 37, 797–803 (2014)
P. Liu, L. Li, Z. Yao, J. Zhou, M. Du, T. Yao, Synthesis and excellent microwave absorption property of polyaniline nanorods coated Li0.435Zn0.195Fe2.37O4 nanocomposites. J. Mater. Sci. 27, 7776–7787 (2016)
A. Phuruangrat, B. Kuntalue, S. Thongtem, T. Thongtem, Synthesis of cubic CuFe2O4 nanoparticles by microwave-hydrothermal method and their magnetic properties. Mater. Lett. 167, 65–68 (2016)
P. Liv, Z. Yao, J. Zhou, Preparation of reduced graphene oxide/Ni0.4Zn0.4Co0.2Fe2O4 nanocomposites and their excellent microwave absorption properties. Ceram. Int. 41, 13409–13416 (2015)
C. Hu, Z. Mou, G. Lu, N. Chen, Z. Dong, M. Hu, L. Qu, 3D graphene-Fe3O4 nanocomposites with high-performance microwave absorption. Phys. Chem. Chem. Phys. 15, 13038–13043 (2013)
M. Fu, Q. Jiao, Y. Zhao, H. Li, Vapor diffusion synthesis of CoFe2O4 hollow sphere/graphene composites as absorbing materials. J. Mater. Chem. A 2, 735–744 (2014)
P. Liu, Z. Yao, J. Zhou, Controllable synthesis and enhanced microwave absorption properties of silane-modified Ni0.4Zn0.4Co0.2Fe2O4 nanocomposites covered with reduced graphene oxide. RSC Adv. 5, 93739–93748 (2015)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51672129 and 51702158) and the Fundamental Research Funds for the Central Universities (Grant No. NS2017036). The authors also thank Dr. Azhar Ali Haidry for his suggestion and revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuo, Y., Yao, Z., Zhou, J. et al. Hierarchical structures based on Li0.35Zn0.3Fe2.35O4/polyaniline nanocomposites: synthesis and excellent microwave absorption properties. J Mater Sci: Mater Electron 29, 922–926 (2018). https://doi.org/10.1007/s10854-017-7989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7989-9