Abstract
In current study, we synthesized PbWO4 using co-precipitation method. Also the effect of three capping agents, polyethylene glycol, cetyltrimethylammonium and sodium dodecyl sulfate on the size of particles, morphology, and photocatalystic properties of nano-structures was investigated. The techniques we employed to characterize the morphological, optical, magnetic and structural properties of as-obtained products were, scanning electron microscopy, vibrating sample magnetometer, energy dispersive X-ray, UV–Vis spectroscopy and X-ray diffraction. The methyl orange method was utilized to assess photocatalytic activity of as-obtained nano-particles under ultraviolet (UV) radiation. Our findings validated that PbWO4 can be decolorized up to maximum value of 97% under 90 min UV irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Supplying fresh water, nowadays, has turned into one of the biggest challenges which all countries, needless to say, they are developed or developing, are facing with. Water scarcity problem can stem from many reasons including, earth’s rising population, overused of water, climate change and increased pollution [1,2,3,4,5]. The importance of free-toxic chemical and diseases-producing microorganism water for human health, has caused many scientists to conduct extensive research to pure water using nano technology. Making use of low dimensional nano particles in water purification, due to their extensive application in different aspects, inspired researchers to give much attention to nano structures [6,7,8,9,10].
One of the most significant aspects of metal nanopaticles, is how pollutants can be removed from environment and water. In other words, making use of photocatalytic and solar energy of nanoparticles, this method has been of advantage to value and efficiency. Changing of living components to carbon dioxide and water without making of remarkable amount of toxics is beneficially of utmost importance [11, 12]. Due to photocatalytic degradation of natural structures, Wolframite-type tungstates of transition metals (MWO4, M = Iron, Cadmium, Zinc, Nickel, Cobalt, rare earth element) have currently received much attention. The materials are widely utilized as, magnetic materials multiferroics, photovoltaic, scinitillator, optical fibers, photoluminescence, super capacitors, scinitillators, molecular precursors to metal tungstate, photocatalyst, and etc [13,14,15]. The previous studies have demonstrated that tungsten oxide (WO3) can be considered as an active photocatalyst material for oxygen evolution, whereas, its activity for hydrogen evolution has not still been proved. That is why different efforts have been made to achieve a series of materials in which MWO3 and MWO4 would be considered as strong photocatalyst compounds. Therefore, the obtained materials displayed attractive technological characteristics for example, ferro elasticity, ionic photoluminescence and ferro elasticity [16,17,18,19]. But to synthesize MWO4, one may perform various methods, such as solid state reaction, solution combustion, sol–gel, solvothermal synthesis, co-precipitation, and hydrothermal synthesis [20,21,22,23]. Of procedures already mentioned, one can expect that co-precipitation method is more advantageous, due to its more simplicity, cost effectiveness, softness chemical synthetic methodology, and fastness in comparison with the rest methods. Also, co-precipitation method does not need specific working conditions, and high temperature to be calcined [24,25,26,27,28]. Therefore, these advantages motivated us to synthesize PbWO4 nanoparticles based on co-precipitation. The synthesizing has been done in distilled water as solvent. In addition, polyethylene glycol, cetyltrimethylammonium and sodium dodecyl sulfate as capping agents, were employed to obtain their influence on the size of particles and morphology of founded nanoparticles.
2 Experimental
2.1 Synthesis of PbWO4 nanostructures
A new simplistic co-precipitation method was employed to prepare nanocrystalline lead tungstate. We followed a step-by-step procedure to make lead tungstate. Firstly, we dissolved 1 mmol of Na2WO4.2H2O in hot water (typically 70 °C). Secondly, it was added drop-by-drop to a 20 ml hot solution (50 °C) containing 1 mmol of Pb(NO3)2 and 3 mmol surfactants with magnetic stirring. We regulated the pH of gained solution in 5–6. Afterwards, under constant magnetic stirring, the resultant solution was heated at 90 °C for 1 h, and then we permitted the system to cool to normal temperature. Subsequently, we collected the obtained precipitation by filtration, and washed several times with absolute ethanol and distilled water. Finally, we dried the product in vacuum at 90 °C. Reaction conditions are available in Table 1.
2.2 Photocatalytic test
To perform photocatalytic activity of PbWO4 nanoparticles, we monitored the degradation process in a quartz photocatalytic reactor when they were exposed to ultraviolet radiation. Firstly, we mixed 0.1 g of nanoparticles with 50 ppm solution of dyes to carry out Photocatalytic degradation. Afterwards, the mixture was positioned in photoreactor under UV light and stirred for half hour at dark, ensuring proper adsorption–desorption equilibrium of the dye molecules on the surface of nanostructures requiring to operate as a photocatalyst efficiently. Then, air was blown into the vessel via a pump maintaining the solution oxygen-saturated for the duration of the reaction. Eventually, the separation of PbWO4 was done from the 5 ml samples and removed from the degraded solution at different time intervals, making use of 10 min centrifuging at 10,000 rpm. A UV–Vis spectrophotometer was used to obtain dye concentration.
2.3 Characterization
The cetyltrimethylammonium bromide (CTAB), polyethylene glycol (PEG), and sodium dodecyl sulfate (SDS) and lead nitrate (Pb(NO3)2), sodium tungstate (Na2WO4.2H2O), were prepared from a well known company, Merck. X-ray diffraction spectra (XRD) were recorded on a Philips-X’PertPro instrument with a Ni-filtered Cu Kα radiation at a scan range of 10 < 2θ < 80. The scanning electron microscopy (SEM) studies were performed on a LEO-1455VP with an energy dispersive X-ray spectroscope. An S-10 4100 Scinco UV–Vis scanning spectrometer was utilized to gain the electronic spectra. Energy dispersive spectra (EDS) were also acquired making use of an XL30 Philips microscope and the magnetic measurements were performed using a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran) under ambient temperatures.
3 Results and discussion
In the few past years through the control of reaction parameters, particle size and control of shape of nanostructures has absorbed much attentiveness. Owing to high dependency of nanoparticles on their shape and particle size [29,30,31,32,33,34], several experiments were carried out to explore the effect of different surfactants on the particle size and morphology of PbWO4 nanoparticles. Using SEM images, we explained the morphologies. Figures 1, 2, and 3 make clear the SEM images of PbWO4 nanoparticles, which were prepared by CTAB, PEG, and SDBS as various capping agents in agreement with sample 1, 2 and 3, respectively.
The effect of presence of PEG, as a polymeric surfactant, on the morphology of synthesized PbWO4 nanoparticles at low temperature, is given in Fig. 1. The SEM images clarified that they are in triangle-like shape and are consist of nanostructures with the average size of 40 nm. The coalesced image of PbWO4 with the average size of 80 nm in the presence of CTAB as a cationic surfactant and rod-like PbWO4 nanoparticles with the average length of 60 nm and width of 2 µm due to using SDS as anionic surfactant have been shown in Figs. 2 and 3 respectively. In addition, because of spatial configuration of SDS surfactant, the morphology of PbWO4 nanoparticles are also in the rod-like shape.
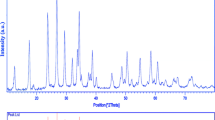
In Fig. 4 XRD, the pattern of PbWO4 nanoparticles has been displayed. In addition, the XRD demonstrated the presence of both tetragonal phase of PbWO4 nanoparticles (JCPDS No. 08-0108, space group: I41/a).The formation of impurity-free PbWO4 nanoparticles, also proved by XRD pattern. The size of crystalline is computed from Scherrer formula as the following:
where β, K and λ are the width of the observed diffraction peak at its half maximum intensity (FWHM), the shape factor which takes a value of about 0.9 and the X-ray wavelength (Cu Kα radiation, equals to 0.154 nm) were about 11 nm for PbWO4 nanoparticles respectively. The analysis of EDS showed the presence of O, Pb and W elements without any impurity (Fig. 5).The measurements of magnetization as a function of the magnetic field were investigated at 300 K, and the hysteric curve with nearly saturated nature at high fields is represented in Fig. 6. Our findings in relation to magnetic properties of PbWO4 nanoparticles, showed that, there magnetic contribution of PbWO4 nanoparticles is small at room temperature. Additionally, the analysis of VSM uncovered that PbWO4 nanoparticles has ferromagnetic properties. The coercivity and saturation magnetization are found to be 80 Orsted and 0.006 emu g−1 and respectively.
Commonly, the band-gap of the nanostructures materials has a major role to play to use photocatalytic applications. The diffuse reflectance spectroscopy (DRS) for PbWO4 nanoparticles has been illustrated in Fig. 7. The band-gap of the samples was measured by the following equation:
where, B, α, Eg, hʋ, and n are material constant, the amount of the absorbance, the optical band gap of the material, the photon energy, and a constant that depends on the type of the electronic transition respectively. Using extrapolating the linear portion of the plots of (αhʋ)2 curve in return hʋ to the energy axis, the energy gap of the samples (Eg) was found. The value of band-gap (Eg) for PbWO4 nanoparticles was 3.2. To investigate the photocatalytic activity, PbWO4 nanoparticles were used. The photocatalytic efficiency was evaluated by the following Eq. (3):
where (mg L−1) is the concentration of MO at any irradiation time t (min) and C0 (mg L−1) is the initial concentration of MO in solution. In Fig. 8 we have shown that, for MO, after 90 min, the degradation rate of PbWO4 nanoparticles was 97, 95, and 84% for PEG, CTAB, and SDS respectively. Therefore, we could find to smallness of recombination of electron–hole possibility in PbWO4 nanoparticles, causing holes in valance band react with OH groups on the surface of nanoparticles. This can turn them into highly reactive OH· radicals. The reaction of produced radicals with MO and its degradation has come in Scheme 1. According to the Langmuir–Hinshelwood kinetics model, the kinetics of the photocatalytic decolourization rate of MO was acquired. This is given in the following Eq. (4):
Utilizing the slope of ln (Co/Ct) in terms of irradiation time t, we quantified. The pseudo-first-order rate constant, kapp (min−1) [35,36,37,38].
4 Conclusions
The removal of MeO dyes from water was done by preparation of PbWO4 nanoparticles. The co-precipitation method was employed to synthesize PbWO4 nanoparticles effectually. The influence of preparation three various surfactants such as PEG, CTAB and SDS on morphology and particle size of products were examined. The homogenous synthesizing of PbWO4 nanoparticles was done, without further impurity. Furthermore, the photocatalytic properties of PbWO4 nanoparticles arise from the value of its band gap. The investigation of the photocatalytic properties of this product was performed by photo-oxidation of methyl orange (MO). The photocatalytic test affirms that the degradation rate of methyl orange, after 90 min irradiation of UV light, picked at approximately 97% for PEG surfactant.
References
M. Rahimi-Nasrabadi, F. Ahmadi, A. Fosooni, J. Mater. Sci. Mater. Electron. 28, 537 (2017)
F. Ahmadi, M. Rahimi-Nasrabadi, M. Behpour, J. Mater. Sci. Mater. Electron. 28, 1531 (2017)
M. Salavati-Niasari, F. Soofivand, A. Sobhani-Nasab, M. Shakouri-Arani, M. Hamadanian, S. Bagheri, J. Mater. Sci. Mater. Electron. (2017). doi:10.1007/s10854-017-7369-5
S.M. Hosseinpour-Mashkani, A. Sobhani-Nasab, J. Mater. Sci. Mater. Electron. 27, 7548 (2016)
S.M. Hosseinpour-Mashkani, A. Sobhani-Nasab, J. Mater. Sci. Mater. Electron. 28, 4345 (2017)
F. Ahmadi, M. Rahimi-Nasrabadi, A. Fosooni, M. Daneshmand, J. Mater. Sci. Mater. Electron. 27, 9514 (2016)
A. Sobhani-Nasab, Z. Zahraei, M. Akbari, M. Maddahfar, S.M. Hosseinpour-Mashkani, J. Mol. Struct. 1139, 430 (2017)
M. Rahimi-Nasrabadi, J. Mater. Sci. Mater. Electron. 28, 2200 (2017)
M. Rahimi-Nasrabadi, F. Ahmadi, M. Eghbali-Arani, J. Mater. Sci. Mater. Electron. 28, 2415–2420 (2017)
M. Rahimi-Nasrabadi, F. Ahmadi, M. Eghbali-Arani, J. Mater. Sci. Mater. Electron. 27, 13294 (2016)
M. Pirhashemi, A. Habibi-Yangjeh, J. Colloid Interface Sci. 491, 216 (2017)
A. Habibi-Yangjeh, M. Shekofteh-Gohari, Sep. Purif. Technol. 184, 334 (2017)
S. Thongtem, S. Wannapop, T. Thongtem, Ceram. Int. 35, 2087 (2009)
C. Wei, Y. Huang, X. Zhang, X. Chen, J. Yan, Electrochim. Acta, 220, 156 (2016).
X.C. Song, E. Yang, R. Ma, H.F. Chen, Y. Zhao, J. Nanopart. Res. 10, 709 (2008)
L. Zhen, W.S. Wang, C.Y. Xu, W.Z. Shao, L.C. Qin, Mater. Lett. 62, 1740 (2008)
M. Rahimi-Nasrabadi, S.M. Pourmortazavi, M.R. Ganjali, A.R. Banan, F. Ahmadi, J. Mol. Struct. 1074, 85 (2014)
K. Adib, M. Rahimi-Nasrabadi, Z. Rezvani, S.M. Pourmortazavi, F. Ahmadi, H.R. Naderi, M.R. Ganjali, J. Mater. Sci. Mater. Electron. 27, 4541 (2016)
W.B. Hu, X.L. Nie, Y.Zh. Mi, Mater. Charact. 61, 85 (2010)
Z. Lou, J. Hao, M. Covivera, J. Lumin. 99, 349 (2002)
M. Bonanni, L. Spanhel, M. Lerch, E. Fuglein, G. Muller, F. Jermann, Chem. Mater. 10, 304 (1998)
A.R. Phani, M. Passacantando, L. Lozzi, S. Santucci, J. Mater. Sci. Mater. Electron. 35, 4879 (2000)
F.S. Wen, X. Zhao, H. Huo, J.S. Chen, E. Shu-Lin, J.H. Zhang, Mater. Lett. 55, 152 (2002)
M. Rahimi-Nasrabadi, J. Mater. Sci. Mater. Electron. 28, 6373 (2017)
M. Rahimi-Nasrabadi, M. Behpour, A. Sobhani-Nasab, M. RangrazJeddy, J. Mater. Sci. Mater. Electron. 27, 11691 (2016)
A. Sobhani-Nasab, H. Naderi, M. Rahimi-Nasrabadi, M.R. Ganjali, J. Mater. Sci. Mater. Electron. 28, 8588 (2017)
M. Rahimi-Nasrabadi, H.R. Naderi, M. Sadeghpour Karimi, F. Ahmadi, S. M. Pourmortazavi, J. Mater. Sci. Mater. Electron. 28, 1877 (2017)
S.M. Hosseinpour-Mashkani, M. Maddahfar, A. Sobhani-Nasab. S. Afr. J. Chem. 70, 44–48 (2017)
M. Ramezani, A. Sobhani-Nasab, A. Davoodi, J. Mater. Sci. Mater. Electron. 26, 5440 (2015)
M. Rahimi-Nasrabadi, S.M. Pourmortazavi, M. Khalilian-Shalamzari, J. Mol. Struct. 1083, 229–235 (2015)
M. Rahimi-Nasrabadi, S.M. Pourmortazavi, M.R. Ganjali, P. Novrouzi, F. Faridbod, M. SadeghpourKarimi, J. Mater. Sci. Mater. Electron. 28, 3325 (2017)
M. Rahimi-Nasrabadi, M. Behpour, A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, J. Mater. Sci. Mater. Electron. 26, 9776 (2015)
H. Reza Naderi, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, M.R. Ganjali, Appl. Surf. Sci. 423, 1025 (2017)
A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, M. Salavati-Niasari, S. Bagheri, J. ClustSci. 26:1305–1318 (2015)
A. Sobhani-Nasab, M. Rangraz-Jeddy, A. Avanes, M. Salavati-Niasari, J. Mater. Sci. Mater. Electron. 26, 9552 (2015)
A. Javidan, M. Ramezani, A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, J. Mater. Sci. Mater. Electron. 26(6), 3813–3818 (2015)
M. Akbari, A. Aetemady, F. Firoozeh, M. Yaseliani, J. Mater. Sci. Mater. Electron. 28, 10245 (2017)
M. Ramezani, A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, J. Mater. Sci. Mater. Electron. 26(7), 4848 (2015)
Acknowledgements
Authors are grateful to council of University of Kashan for providing financial support to undertake this work. This work was supported by Council of University of Kashan by Grant Agreement No. 682151/1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pourmasoud, S., Eghbali-Arani, M., Ahmadi, F. et al. Synthesis, characterization, and morphological control of PbWO4 nanostructures through precipitation method and its photocatalyst application. J Mater Sci: Mater Electron 28, 17089–17097 (2017). https://doi.org/10.1007/s10854-017-7635-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7635-6