Abstract
Barium hexa ferrite (BaFe12O19) nanoparticles were synthesized via a facile precipitation method in solvent of water. Then titanium dioxide nanoparticles as effective photo-catalyst were prepared via a simple sol–gel method. Finally BaFe12O19–TiO2 (50:50%) nanocomposites were made by a fast chemical procedure. The effect of concentration, temperature and precipitating agent on the morphology and particle size of the products was investigated. The prepared products were characterized by X-ray diffraction, scanning electron microscopy, and Fourier transform infrared spectroscopy. Vibrating sample magnetometer shows the ferromagnetic property of the ferrite nanostructures. The photocatalytic behaviour of BaFe12O19–TiO2 (50:50%) nanocomposites was evaluated using the degradation of azo dyes (acid black, acid violet, acid blue and methyl orange) under ultraviolet light irradiation. The results introduce a nanocomposite with applicable magnetic and photocatalytic performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hexagonal ferrites and their substituted derivatives have attracted considerable attention and they show very high uniaxial magnetic anisotropy so they can be used as a permanent magnetic material, which for some applications can compete technically and economically, with metallic permanent magnets [1,2,3]. BaFe12O19 is one of the most important hard magnetic materials, with high Curie temperature, excellent chemical stability, corrosion resistivity excellent high-frequency response and narrow switching field distribution it widely used for permanent magnets, magnetic recording, microwave absorbers. Barium ferrite is a ferromagnetic material, which has a collinear five-sublattice magnetic structure with easy axis parallel to the c-axis of the hexagonal unit cell. Its crystal structure can be divided in several blocks according to the stacking form of closely packed oxygen ions [4,5,6].
Alternatively, wet synthesis procedures have been developed. Usually, these procedures involve the coprecipitation of ferrihydrite, or amorphous ferric hydrous oxide, and barium hydroxide, followed by calcination to relatively low temperatures. High-frequency electromagnetic properties of the barium ferrite family have also been investigated from the view point of the application to the high-frequency active devices or electromagnetic wave absorbers [7].
Growing social concern about the impact of different chemicals on the environment has focused attention on finding ways for more effective pollution abatement methods. Following this, one of the more promising techniques is to use a photocatalytic route to oxidize such chemicals [8, 9]. The strong oxidizing power of the photo-generated holes, the chemical inertness, and the non-toxicity of TiO2 has also made it a superior photocatalyst. This material is known to exist in several forms, among them the most abundant are anatase, rutile and brookite [8]. TiO2 is superior than other substances because of its high photocatalytic activity and chemical stability in aqueous solution under UV light irradiation. Highly dispersed fine particles on porous support materials and suspended fluids in liquid medium. When the suspension TiO2 is used for photodegradation of organic compounds, the suspended TiO2 must be separated after each reaction. This problem could be avoided by using the TiO2 on the magnetic cores [9].
2 Experimental
2.1 Materials and methods
Ba(NO3)2, Fe(NO3)3 ⋅ 9H2O, NaOH, NH3 32%, tetraisopropyl orthotitanate (TTIP), polyvinylpyrrolidone, starch, gelatin, glucose, salicylic acid, nitric acid, methanol, ethanol and acetone were purchased from Merck or Aldrich and all the chemicals were used as received without further purifications. A multi-wave ultrasonic generator (Bandeline MS 73), with a converter/transducer and titanium oscillator, operating at 20 kHz with a maximum power output of 150 W was used for the ultrasonic irradiation. Room temperature magnetic properties were investigated using vibrating sample magnetometer (VSM) device, by Meghnatis Kavir Kashan Company (Iran) in an applied magnetic field sweeping between ± 10,000 Oe. X-ray diffraction (XRD) patterns were recorded by a Philips, X-ray diffractometer using Ni-filtered CuKα radiation. Scanning electron microscopy (SEM) images were obtained using a LEO instrument model 1455VP. Prior to taking images, the samples were coated by a very thin layer of Pt (using a BAL-TEC SCD 005 sputter coater) to make the sample surface conductor and obtaining a better contrast.
2.2 Synthesis of BaFe12O19 nanoparticles
0.1 g of Ba(NO3)2 and 1.85 g of Fe(NO3)3 ⋅ 9H2O were dissolved in 100 ml of deionized water. 30 ml of NaOH solution (1 M) was then slowly added to the solution until reaching pH to about 10. A brown precipitate was then centrifuged and washed with distilled water. Eventually the obtained precipitate was calcined at 800 °C and its color goes from brown to black. Figure 1 shows the schematic diagram for experimental setup for nanoparticle and nanocomposite preparation used in the precipitation procedure.
2.3 Synthesis of TiO2 nanoparticles
0.25 g of TTIP was added to 10 ml of methanol. Then 1 ml of distilled water was added to the solution. In this level white sol was formed. Sol was stirred for 20 min, then 0.2 ml of HNO3 was added to the sol until pH reaching around 2. After 90 min stirring gel was formed. Finally the obtained powder was calcined at 500 °C.
2.4 Synthesis of (BaFe12O19–TiO2 50:50%) nanocomposite
0.1 g (50%) of BaFe12O19 was dispersed in 10 ml of methanol. Then 0.25 g of TTIP (Yield of TiO2:0.1 g, 50%) was added to the mixture containing BaFe12O19. Then 1 ml of distilled water was added to the mixture and was stirred for 20 min. Then 0.2 ml of HNO3 was slowly added to the aqueous solution and was stirred for 90 min, until white gel was formed. After 1 h the gel was placed in the oven (70 °C) to dry. Finally obtained powder was calcined at 500 °C.
2.5 Photo-catalytic degradation process
5 ml of the dye solution (10 ppm) was used to determine the photocatalytic activity. For degradation of 10 ml solution 0.1 g of catalyst was used. The solution was irradiated under UV lamp (8 W) which was placed in a quartz pipe. The nano-samples were centrifuged and dye concentration was specified by UV–Vis spectrometer.
3 Results and discussion
Scanning electron microscopy was employed for estimation of morphology and particle size of the products. Figure 2 shows SEM images of BaFe12O19 nanoparticles prepared without using any surfactant and capping agent, results confirm that mono-disperse nanoparticles with average size less than 40 nm were synthesized.
Figure 3 illustrate SEM photograph of barium ferrite nanoparticles synthesized with salicylic acid as a green capping agent, images confirm that mono-disperse nanoparticles with average size around 50 nm were synthesized. Salicylic acid as a neutral surfactant leads to better nucleation in comparison to growth stage.
Gelatin as another green capping agent was used, Fig. 4 illustrates SEM images of BaFe12O19 nanoparticles synthesised at presence of Gelatin, it depicts that agglomerated nanoparticles with mediocre size around 100 nm were achieved. It seems that carbohydrate chains remain around the nanoparticles and adsorbed on the surface and at the final bigger particles were obtained.
Polyvinylpyrrolidone as another conventional surface active agent was used and outcomes approve that it is a unsuitable capping agent for preparation of barium ferrite via precipitation method. SEM photograph of barium ferrite nanoparticles synthesised with PVP is illustrated in Fig. 5 which bulk and agglomerated product was obtained.
Figure 6 shows SEM images of BaFe12O19 nanostructures synthesised with starch, by applying this safe and cost-effective sugar, mono-disperse nanoparticles with average size less than 35 nm were synthesized.
Concentration effect on the morphology and size of magnetic product was investigated. Figure 7 illustrate SEM photograph of barium ferrite at 200 ml of water, mono disperse nano-rods and rice like product with diameter size about 50 nm was prepared.
Temperature influence on the shape and size was also examined, SEM images of BaFe12O19 nanoparticles achieved at 800 °C is shown in Fig. 8, mono-disperse nanoparticles with mediocre size around 50 nm were prepared.
Reaction with salicylic acid was chosen as basic reaction and effect of various parameters on the shape and size was also examined. Calcination temperature influence on the size was investigated. Figure 9 illustrates SEM photograph of barium ferrite nanoparticles calcined at 200 °C. Mono-disperse magnetic nanoparticles with size lower than 50 nm was achieved.
SEM images of BaFe12O19 nanoparticles calcined at 500 °C are shown in Fig. 10. Mono-disperse nanoparticles around 50 nm were synthesized.
Figure 11 illustrates SEM photographs of titanium dioxide at ethanol nanoparticles that show nanoparticles with size about 50 nm were prepared.
The effect of solvent on the shape was surveyed, SEM images of TiO2 at methanol (Fig. 12) confirm mono-disperse nano spheres with diameter size about 50 nm were obtained.
Photocatalyst and magnetic nanocomposite is depicted in Fig. 13. SEM images of BaFe12O19–TiO2 (50:50%) nanocomposites approve by adsorption and synthesise of titanium dioxide on the magnetic core, nanoparticles with dimensions less than 100 nm were prepared.
Figure 14 shows the Fourier transform infrared (FT-IR) spectrum of the as-prepared BaFe12O19 nanoparticles, the absorptions band at 430, 580 and 619 cm−1 are assigned to the stretching mode of Fe–O and Ba–O. The spectrum exhibits broad absorption peak around 3479 cm−1, corresponding to the stretching mode of O–H group of hydroxyl group and the band near 1431 cm−1 is assigned to vibration mode of aromatic ring C=C bond due to the adsorption of salicylic acid on the surface of nanoparticles.
FT-IR spectrum of the TiO2 nanoparticle is shown in Fig. 15 The absorptions band at 345 and 621 cm−1 are assigned to the stretching mode of Ti–O bonds. Figure 16 illustrates the FT-IR spectrum of the as-prepared BaFe12O19–TiO2 (50:50%) nanocomposites. Strong absorption band approves presence of magnetic nanoparticles. All absorptions band at 300 and 553 cm−1 are assigned to the stretching mode of Fe–O, Ba–O and Ti–O bonds. Absorptions around 3414 cm−1 are corresponding to the O–H group.
The structure and composition of the BaFe12O19 nanoparticles was investigated. Figure 17 shows XRD pattern of sample including barium ferrite nanoparticles. The XRD pattern of BaFe12O19 reveals the typical diffraction pattern of pure Hexagonal phase (JCPDS No.: 07-0276) with P63-mmc space group which is consistent with pure barium ferrite.
XRD pattern of TiO2 nanoparticles was also investigated in Fig. 18. Titanium dioxide reveals the typical diffraction pattern of agreement with pure tetragonal TiO2 phase (JCPDS No.: 02-0387) with I41-amd and (JCPDS No.:0 1-1292) with P42-mnm space group which has.
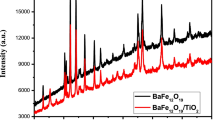
Figure 19 depicts XRD pattern of BaFe12O19–TiO2 (50:50%) nanocomposite. Presence of both hexagonal phase and pure tetragonal phase was confirmed in the XRD pattern.
The crystalline sizes from Scherrer equation, Dc = Kλ/βCosθ, was calculated, where β is the width of the observed diffraction peak at its half maximum intensity (FWHM), K is the shape factor, which takes a value of about 0.9, and λ is the X-ray wavelength (CuKα radiation, equals to 0.154 nm). The average crystalline size for BaFe12O19, TiO2, BaFe12O19–TiO2 (50:50%) nanoparticles were found to be about 19, 21 and 29 nm respectively.
Room temperature magnetic property of ferrite that was prepared by salycilic acid was studied using VSM instrument and is shown in Fig. 20. The result indicates that, the sample exhibit ferromagnetic property. Saturation magnetizations around 36 emu/g and coercivity about 1650 Oe have been achieved.
Figure 21 shows VSM curve of BaFe12O19 nanoparticles prepared at diluted solvent (200 ml of water instead of 100 ml), the product show ferromagnetic property. Saturation magnetizations around 45 emu/g and coercivity about 1285 Oe have been achieved.
Figure 22 depicts magnetization curve of BaFe12O19–TiO2 (50:50%) that also exhibits ferromagnetic behaviour with a coercivity of about 2700 Oe and saturation magnetization of 18 emu/g.
The magnetic property of the prepared nanocomposites is an essential characteristic of a heterogeneous nanocomposite since materials with this magnetic behaviour have low tendency in inter-particles agglomeration caused by dipole–dipole interaction in comparison with ferromagnetic nanocomposites. The results also indicate that nanocomposite formation give rise to coercivity enhancement.
The mechanism of photo-degradation of toxic azo-dyes under ultraviolet irradiation in the presence of titanium dioxide as an effective photo-catalyst with an indirect band gap about 3.2 eV is shown in Fig. 23. Acid black, acid blue, acid violet and methyl orange as typical organic pollutants were employed as targets because of the relative stability of their molecular structure. UV–Vis absorption of TiO2 is shown in Fig. 24 respectively. The as-prepared nanocomposite has the potential to be applied to improve environmental problems associated with organic and toxic water pollutants.
Maximum absorption peaks (λmax) of organic dyes that were used for degradation under UV light are prepared from UV–Vis absorption spectra and were confirmed by scientific literature. The photo-catalytic activity of the nanocomposite was evaluated by monitoring the degradation of organic dyes in an aqueous solution, under UV irradiation. The changes in the concentration of dye are illustrated in Fig. 25 acid blue, acid violet, methyl orange and acid black were degraded at 15, 40, 60 and 30 min respectively by BaFe12O19–TiO2 (50:50%) nanocomposite. As time increase; more and more dyes are adsorbed on the nanoparticles catalyst, until the absorption peaks (λmax) of acid black, methyl orange, and acid blue decrease and vanish around 60 min. The dyes concentration decreased rapidly with increasing UV–irradiation time organic dyes decompose to carbon dioxide, water and other less toxic or nontoxic residuals [10,11,12,13,14,15,16].
4 Conclusions
In conclusion synthesis, characterization, and photocatalytic activity of BaFe12O19, TiO2 and BaFe12O19–TiO2 (50:50%) nanocomposite were reported. VSM confirmed that nanoparticles and nanocomposite exhibit ferromagnetic behaviour. The photocatalytic behaviour of BaFe12O19–TiO2 (50:50%) nanocomposite was evaluated using the degradation of four various azo dyes under UV light irradiation. The results show that precipitation method is a suitable method for preparation of BaFe12O19–TiO2 (50:50%) nanocomposites as a candidate for photocatalytic applications.
References
T. Gonzalez-Carreno, M.P. Morales, C.J. Serna, Mater. Lett. 43, 97 (2000)
Z. Haijun, L. Zhichao, M. Chengliang, X. Yao, Z. Liangying, W. Mingzhong, Mater. Sci. Eng. B 96, 289 (2002)
X. Liu, J. Wang, L.-M. Gan, S.-C. Ng, J. Magn. Magn. Mater. 195, 452 (1999)
G. Mu, C. Na, P. Xifeng, S. Haigen, M. Gu, Mater. Lett. 62, 840 (2008)
H. Zhang, Z. Liu, C. Ma, X. Yao, L. Zhang, M. Wu, Mater. Chem. Phys. 80, 129 (2003)
S.E. Jacobo, C. Domingo-Pascual, R. Rodriguez-Clemente, M.A. Blesa, J. Mater. Sci. 32, 1025 (1997)
N. Koga, T. Tsutaoka, J. Magn. Magn. Mater. 313, 168 (2007)
K.M. Reddy, V.M. Sunkara, A.R. Reddy, Mater. Chem. Phys. 78, 239 (2003)
R.S. Sonawane, S.G. Hegde, M.K. Dongare, Mater. Chem. Phys. 77, 744 (2003)
D. Ghanbari, S. Sharifi, A. Naraghi, G. Nabiyouni, J. Mater. Sci. Mater. Electron. 27, 5315 (2016)
A. Shabani, G. Nabiyouni, J. Saffari, D. Ghanbari, J. Mater. Sci. Mater. Electron. 27, 8661 (2016)
S. Masoumi, G. Nabiyouni, D. Ghanbari, J. Mater. Sci. Mater. Electron. 27, 9962 (2016)
S. Masoumi, G. Nabiyouni, D. Ghanbari, J. Mater. Sci. Mater. Electron. 27, 11017 (2016)
G. Rezaei, G. Nabiyouni, D. Ghanbari, J. Mater. Sci. Mater. Electron. 27, 11339 (2016)
K. Hedayati, M. Goodarzi, M. Kord, Main Group Met. Chem. 39, 183 (2016)
K. Hedayati, M. Kord, M. Goudarzi, D. Ghanbari, S. Gharigh, J. Mater. Sci. Mater. Electron. 28, 1577 (2017)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebrahimi, Z., Hedayati, K. & Ghanbari, D. Preparation of hard magnetic BaFe12O19–TiO2 nanocomposites: applicable for photo-degradation of toxic pollutants. J Mater Sci: Mater Electron 28, 13956–13969 (2017). https://doi.org/10.1007/s10854-017-7245-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7245-3