Abstract
Low-cost Mn2+-doped willemite (α-Zn2SiO4:Mn2+) based glass–ceramics were synthesized by conventional melt–quenching technique using waste soda lime silica (SLS) glasses, zinc oxide (ZnO) and Manganese oxide (MnO) as precursors. The effect of different MnO percentage doping on physical, structural, optical and luminescent performance α-Zn2SiO4:Mn2+ based glass–ceramics were comprehensively studies in this work. The presence of α-Zn2SiO4:Mn2+ crystal phase and microstructure was confirmed by X-ray diffraction and field emission scanning electron microscopy spectroscopy. From the Scherrer’s formula, α-Zn2SiO4:Mn2+ have an average crystallite size of 30–40 nm, respectively. Fourier transform infrared reflection spectroscopy displays the structural growth of α-Zn2SiO4:Mn2+ crystal. The green emission centered at about 527 nm from the α-Zn2SiO4:Mn2+ crystal exhibit a resulted from 4T1–6A1 energy transition of Mn2+ ions. Intense emissions of Mn2+ ions at 260 nm excitation were occurs may be caused by the increase of Mn2+ ions into α-Zn2SiO4 crystal structure with lower phonon. Based on the results achieved, this low-cost α-Zn2SiO4:Mn2+ based glass–ceramic exhibit a huge potential to act as a green phosphor in opto-electronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, production of new vitreous phosphor materials for opto-electronic industries is grabbing many attentions from researchers and the industry production. The three main colors red, green and blue can be gained from upconversion luminescence of rare earth and transition metal ions [1]. Nevertheless, the discharge and control of the relative intensities of the three main colors are mandatory. Thus, it is essential to develop novel materials host capable of multicolor upconversion and scrutinize its upconversion mechanisms of rare earth and transition metal ions engaged. Furthermore, the chemical composition, particle size distribution and surface morphology must be optimized in order to produce the high quality phosphor.
Willemite (Zn2SiO4) is belongs to cyclo-silicates with a three-dimensional character with a high thermal stability and been expanded by doping diverse divalent metal cations to use as phosphors and photocatalysts [2]. Its superior properties such as chemical stability, transparency in the ultraviolet (UV) and visible range make Zn2SiO4 is ideal host material for both rare earth and transition metal ions [3]. Furthermore, Zn2SiO4 is polymorphic and may exist in the three different phase (α, β and γ phases). The β and γ phases Zn2SiO4 are metastable and transforms to the α-phase at high temperatures [4,5,6].
Abundant attention has been paid to transition metal and rare earth ion-doped Zn2SiO4 as efficient luminescent materials [7]. Among the phosphor, α-Zn2SiO4 has been labeled as a capable host matrix for a variety of transition metal and rare earth ions for effective luminescence in the red, yellow and green spectral zones for lighting and display devices [8]. The β-Zn2SiO4 phase is a metastable crystal structure and emits yellow emission whiles α-Zn2SiO4 is a stable structure and displays green emission [9]. Besides, α-Zn2SiO4 is a well-known host material for the phosphors in light emitting devices [10]. The α-Zn2SiO4 green phosphor gain lot of particular interest because of its high luminescence efficiency, high photostability (especially under UV excitation), stability to moisture sensitivity, low chemical inertness, high saturated color, and long life span [11,12,13,14]. These properties make it a best material for cathode ray tubes (CRT), fluorescent lamps, vacuum fluorescent displays, and plasma display panels (PDP) [15, 16].
Among the transition metal ions, Mn2+ ions are vital activator in producing red-green emission for the host phosphor lattices because of its 4T1→6A1 transition [17]. From the viewpoint of applicable applications, the discharged wavelengths of Mn2+ ions are situated at the lesser energy region. A recent report on Zn2SiO4:Mn2+ materials demonstrate a steady improvement of green light emission with the formation of Mn2+ ion pairs. Recently, α-Zn2SiO4:Mn2+ is utilize in high volume for the most advanced televisions and electronic devices due to its high luminescence efficiency, great color purity, very in chemical and thermal stabilities. At the present, α-Zn2SiO4:Mn2+ have been classified as one of the most practical and attractive materials used in opto-electronic devices [18]. However, from the crystal-chemical point of view, the structural properties of α-Zn2SiO4:Mn2+ are still questionable. According to the report, the most intense photoluminescence emission corresponds to the highest concentration of Mn2+ ion pair centres [19]. On the other hand, there are only a small number of reports on the synthesis and structural characterization of α-Zn2SiO4:Mn2+ based glass–ceramics.
In this study, α-Zn2SiO4:Mn2+ based glass–ceramics has been derived from MnO-doped ZnO–SLS glass system by conventional melt-quenching technique using waste soda lime silica (SLS) glass as silica source, zinc oxide (ZnO) and manganese oxide (MnO) as precursors followed by heat treatment process at 700 °C for 2 h. The effects of the manganese concentration, crystal structure and crystallite size on the physical, structural, optical and photoluminescence performance of these glass–ceramic were investigated.
2 Methodology
2.1 Sample preparation
A series of precursor glass sample with the chemical formula of x(MnO)100-x[50(ZnO)50(SLS)], where x = 0, 1, 2, 3 and 10 wt% was successfully prepared from appropriate amounts of waste soda lime silica (SLS) glasses as a source of silica, analytical grade of zinc oxide (99.99%, Alfa Aesar) and manganese oxide (99.99%, Alfa Aesar) powders. About 30 gmixtures of raw materials were mixed and transfer to the 50 ml alumina crucible and melt in an electric furnace at 1400 °C for 2 h in air. The melts are then quenched by pouring directly into cold water to produce transparent glass frits. Glass frits are used as precursors for crystallization in order to obtain well crystallized products with minimum porosity. The glass frits were dried and ground using agate mortar and pestle to produce powder in size ~45 µm. Polyvinyl Alcohol (PVA) with 1.75 wt% is added to the fine powders which acts as the binders and later have been compressed to form disk pellets at a pressure of 5 tons. The as-prepared glass pallets were heat-treated at 700 °C for duration 2 h to obtain α-Zn2SiO4:Mn2+ based glass–ceramics.
2.2 Characterization
The samples were characterized by XRD (PANalytical (Philips) X’Pert Pro PW 3050/60) with Cu Kα radiation within the range of 2θ = 0°–80°. The microstructure of the samples was investigated using FESEM (FEI Nova NanoSEM 30 Series). To determine the bonding structure, FTIR spectroscopy (Perkin Elmer (US) Spectrum 100 spectrometer) with Universal attenuated total reflectance (ATR) accessory was applied. The infrared spectra were recorded in the range of wavenumber of 400–4000 cm−1. The absorption spectra of the glasses were obtained with Shimadzu UV–VIS–NIR Spectrophotometer in the range of 250–700 nm. The absorbance of light energy that excites electrons from the ground state to the first singlet excited state of the samples was being absorbed. These measurements are made on glass and glass ceramic powder with size ≤63 μ that have been compressed in a specific holder. However, there was a limitation when using this technique which is the particle size need to be small and fine about <20 µm so that the precise data can be achieved. The application of the Tauc plot method has been used widely to determine band gap energy by using the diffuse reflectance spectrum as shows;
where α is absorption coefficient, h is Planck’s constant, v is frequency of vibration, B is proportional constant and E opt is the optical band gap. From the specific software inside the UV–Vis computer, the obtained diffuse reflectance spectrum was converted to Kubelka–Munk function. Thus, the vertical axis was converted to quantity F(R∞), which is proportional to the absorption coefficient. The α in the Tauc equation was substituted with F(R∞) hence the relational expression becomes;
In order to find the type of transition, the E opt values are calculated by extrapolation of the linear parts of \({\left( {\alpha hv} \right)^{1/n}}\) vs. hv curves to \({\left( {\alpha hv} \right)^{1/n}}={ }0\) for different values of transition. Here, the unit of hν is eV (electron volts), and its relationship to the wavelength λ (nm) becomes hv = 1239.7/λ. Tehn the hν value at the point of intersection of the tangent line and the horizontal axis is determined the \({E_{opt}}\)values. The type of transition can be obtained from the value of n. PL emission spectra measurement of the samples were recorded at room temperature by a fluorescence spectrophotometer (Perkin-Elmer LS 55) comprising of 450 W xenon lamp as the excitation source in standard room temperature with an excitation wavelength of 260 nm for all samples.
3 Results and discussion
3.1 Phase composition and crystal structure
The chemical composition of the glass samples were analyzed using EDXRF. The chemical composition obtained was given in the Table 1. Eight oxide element and small percentage of unknown oxide were detected in the glass samples. Elements like SiO2, CaO, Na2O, Al2O3, K2O and MgO are originated from SLS waste. From Table 1, the increases in MnO addition have resulted in a reduction to other elements in glass samples.
Figure 1 was plotted to study the effect of MnO concentration towards linear shrinkage of samples sintered at 700 °C for 2 h. From the graph, it was clearly observed that the linear shrinkage increased with increasing in MnO concentration. The goal of sintering temperature is to convert a porous sample into dense material [20]. There are two possibilities for the samples undergo sintering process whether coarsening or densification. If the coarsening controls, the sizes of the pores and grains of the material will increase with time. However, if the densification dominates, the pore sizes will decrease and tend to shrink [21]. Thus, in this work, densification are dominates since the samples display the shrinkage behavior.
The density of samples prepared at different MnO weight percentage were plotted as a function of different MnO concentration are shows in Fig. 2, respectively. From the graph it is revealed that the density of the samples increased from 2.573 to 2.696 g/cm3 with the progression of MnO content after the heat treated at 700 °C for 2 h. An increased trend in density is due to the presence of the heavier Mn atomic mass (54.938 a.m.u) after Zn (65.390 a.m.u) compared to any element that present in the SLS glasses such as Si (28.086 a.m.u), Ca (40.078 a.m.u) and Na (22.989 a.m.u).
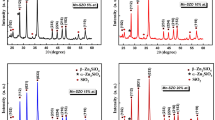
Figure 3 illustrates the XRD pattern of samples sintered at 700 °C, respectively. Phase analysis indicates that all the XRD diffraction peaks position in Fig. 3 match well with those of the standard pattern reported by the Joint Committee on Powder Diffraction Standards (JCPDS) data card number 37-1485, which can be assigned to α-Zn2SiO4:Mn2+ (rhombohedral structure, space group: R \(\stackrel{-}{3}\)) with lattice parameter (a = 13.9381, b = 13.9381, c = 9.3100 Å and α = 90.00°, β = 90.00°, γ = 120.00°) [22]. As can be seen in the Fig. 3, two phases can be observed; the minor ZnO phase (JCPDS 36-1451) and α-Zn2SiO4:Mn2+ (JCPDS 37-1485) as a major phase in the glass–ceramic structure. XRD results of the samples matches very well with the α-Zn2SiO4:Mn2+ phase and it can be concluded that α-Zn2SiO4:Mn2+ stable phase can be obtain after sintering process at 700 °C for 2 h. All of the samples exhibited similar XRD patterns which no differences in crystallinity phases were detected in the samples with varying amounts of MnO. The XRD patterns of the samples revealed the formation of α-Zn2SiO4:Mn2+ phase for all sample compositions. However, it is observed a slight shift in the XRD peaks, due to variation of the lattice parameters in the solid solutions with increasing Mn2+ content, cell volume variations are small due to the very close ionic radii: 0.60 Å for Zn2+ and 0.66 Å for Mn2+ (CN = 4) [23]. At higher MnO concentration, the intensity ZnO phase tend to decrease which indicate that the Mn2+ can be completely build into the α-Zn2SiO4 host lattice by substitution for the Zn2+. However, coexistence of both ZnO and α-Zn2SiO4:Mn2+ phases show that the solid state reaction is not completed. The intensity of the diffraction peaks increased with the increase of MnO content up to 3 wt% and suddenly decreased when the content of the dopant is to high (10 wt%). This trend is resulted by a loss of crystallinity due to change and rearrangement of lattice distortion. After introduction of Mn2+ ions into the willemite lattice, a strain is induced into the system which leads to the alteration of the lattice periodicity and decrease its crystal symmetry. By using FWHM values of the maximum diffraction peak of α-Zn2SiO4:Mn2+ phase, the average crystallite size is calculated by converting the data using the Scherrer’s formula. From the calculation, α-Zn2SiO4:Mn2+ phase exhibited an average crystallite size of 30–40 nm.
The morphology of the samples in the Fig. 4 shows that the crystallized grains are still in irregular shape and aggregated after heat treated at 700 °C for 2 h. The micrographs reveal that there are crystals growth on the surface of the samples as an indication of formation of ZnO and α-Zn2SiO4:Mn2+ crystals. The micrograph shows that the particle size varies from 600 to 700 nm. This is in a good agreement with Lenggoro and coworkers as they stated that samples prepared using methods such as solid-state, co-precipitation and hydrothermal are irregular in shape and size. Other researcher who also prepared α-Zn2SiO4:Mn2+ using solid-state method reported that their particles were composed of rectangular shaped grains with relatively large size of 1–2 μm [24].
The FTIR spectra of undoped and Mn-doped Zn2SiO4 based glass–ceramic that have been sintered at 700 °C are shown in Fig. 5, respectively. All the peaks appeared on each samples are simplified in the Table 2. Figure 5 shows FTIR spectra of undoped and Mn-doped Zn2SiO4 that have been sintered at 700 °C for 2 h. The FTIR band observed is more intense. Vibrational mode at ~570 cm−1 which corresponds to Zn–O–Si symmetric stretching vibrational mode is more defined at low percentage of manganese. No significant difference can be detected except for the disappearance of band at ~690 cm−1 which attributed to ZnO4 asymmetric stretching vibrational mode for the sample that have been doped with 10 wt% of manganese. The observed appearance of both SiO2, ZnO4 and Zn–O–Si bands indicate that the formation of Zn2SiO4 phase was formed.
3.2 Optical properties
Absorption spectra of undoped and Mn-doped willemite based glass–ceramic samples sintered at four different temperatures were recorded in the region 250–700 nm are demonstrated in Fig. 6. It is also can be concluded that the intensity of the absorption spectra increased with increasing MnO concentration. In this study, fundamental absorption edge of the glass–ceramic samples arises because of the indirect transition. Optical band gap energy is agreed to be [25]:
where α is the optical absorption coefficient obtained from UV–Vis measurement, C is the constant independent of photon energy (hv) while E g shown is the indirect allowed optical band gap energy. Based on the plot of \({\left( {\alpha hv} \right)^{1/2}}\)against hv, the optical band gap energy, E g, of the samples was acquired by extrapolating the linear regions of the plot to \({\left( {\alpha hv} \right)^{1/2}}={ }0\). The plot of \({\left( {\alpha hv} \right)^{1/2}}\) against (hv) shows a linear dependence. The band gap energy (E g ) was determined by extrapolating the linear part of the spectrum \({\left( {\alpha hv} \right)^{1/2}}\)curve towards the (hv) axis in Fig. 7. However, the fundamental absorption edge of the samples is assumed to be direct transition for glass–ceramic samples.
where α is the optical absorption coefficient, C is the constant independent of photon energy (hv) and E g is the direct allowed optical band gap energy. The band gap energy (E g ) is determined by extrapolating the linear part of the spectrum \({(\alpha hv)^2}\)curve towards the (hv) axis. The band gap energy of the samples were obtained when the point of extrapolation of the linear part encounter the abscissa axis and is shown in Fig. 7. The obtained E g values for all glass and glass–ceramic samples are compiled in Table 3. Based on the result on the shown in Table 3, it can be concluded that the optical band gap energy of the samples will drop as the percentage of doping rises. Drop in the values of band gap after acquiring Mn is mainly because the Mn defects in the bands. These MnO defects will result in the absorption of incident photons and causes effect in the band gap. Hence, the electrons which are responsible to move from valence band to the conduction band would acquire more energy. The decrease in optical band gap value was found to be in a good agreement with other researcher [26,27,28].
3.3 Photoluminescence properties
Figure 8 illustrates the photoluminescence emission spectra of undoped and Mn-doped willemite based glass–ceramics sintered at 700 °C, respectively. The emission spectra were measured at an excitation wavelength of 260 nm. These green emission has been assigned to an electronic transition of 4T1–6A1 peaking at the wavelength 527 nm in which is a parity forbidden emission transition of Mn2+ ions [29]. From the graph, the emission intensity of the prepared glass–ceramics shows increasing trend as the percentage of MnO doping content increased until 3 wt% of Mn-dopant content and decreased significantly at the 10 wt% of Mn-dopant content. Due to high percentage of MnO doping, the extra peak of Mn2SiO4 as a side product; not detectable in XRD patterns due to a small crystal amount of less than limitation of XRD measurement [30]. It can be concluded that the impurity Mn2SiO4 crystal can give a negative effect on overall material properties such as PL quenching behaviors at higher concentrations and temperatures, and weakening of spin–spin interaction [31]. From this results, we can conclude that more than 3 wt% of MnO doping content is not effective to enhance its emission intensity. Excessive doping may bring out many defects which can affect efficient energy transfer from host matrix to activated Mn2+ ions [31].
4 Conclusion
In summary, Mn2+-doped willemite (α-Zn2SiO4:Mn2+) based glass–ceramic derived from MnO doped ZnO–SLS glass system have been successfully prepared using melt–quench method followed by heat treatment process. The α-Zn2SiO4:Mn2+ crystal phase was obtained after sintering process at 700 °C for 2 h. The effect of different MnO percentage doping on physical, structural, optical and luminescent performance have been studied in detail. The structural characterization verifies the crystalline phases of glass–ceramic and formation of α-Zn2SiO4:Mn2+ and ZnO crystal phases. Investigations of optical band gap show that optical band gap decreased from 4.09 to 2.58 eV as we move towards higher concentration of MnO. The emission wavelength was obtained at 527 nm which is attributed to green emission. The emission intensity amplified as the dopant concentration increased to 3 wt% and decreased at 10 wt% of dopant concentration due to the concentration quenching phenomenon.
References
C.R. Ronda, T. Jüstel, H. Nikol, Rare earth phosphors: fundamentals and applications. J. Alloys Compd. 275, 669–676 (1998)
D. Behal, B. Röska, U. Gattermann, A. Reul, S.H. Park, Structure analysis of a Mn-doped willemite-type compound, H0.12(Zn1.89(3)Mn0.05(1)(0.06)Si1.00(1)O4.. J. Solid State Chem. 210(1), 144–149 (2014)
A.G. Joly, W. Chen, J. Zhang, S. Wang, Electronic energy relaxation and luminescence decay dynamics of Eu3+ in Zn2SiO4:Eu3+ phosphors. J. Lumin. 126(2), 491–496 (2007)
M. Takesue, H. Hayashi, R.L. Smith, Thermal and chemical methods for producing zinc silicate (willemite): a review. Prog. Cryst. Growth Charact. Mater. 55(3), 98–124 (2009)
M.H.M. Zaid, K.A. Matori, H.A.A. Sidek, M.K. Halimah, Z.A. Wahab, Y.W. Fen, I.M. Alibe, Synthesis and characterization of low cost willemite based glass–ceramic for opto-electronic applications. J. Mater. Sci. 27(11), 11158–11167 (2016)
C.E. Rivera-Enríquez, A. Fernández-Osorio, J. Chávez-Fernández, Luminescence properties of α-and β-Zn2SiO4:Mn nanoparticles prepared by a co-precipitation method. J. Alloys Compd. 688, 775–782 (2016)
F. Su, B. Ma, K. Ding, G. Li, S. Wang, W. Chen, A.G. Joly, D.E. McCready, Luminescence temperature and pressure studies of Zn2SiO4 phosphors doped with Mn2+ and Eu3+ ions. J. Lumin. 116(1), 117–126 (2006)
B.C. Babu, & S. Buddhudu, Dielectric properties of willemite Zn2SiO4 nano powders by sol–gel method. Physics Procedia 49, 128–136 (2013)
Y. Jiang, J. Chen, Z. Xie, L. Zheng, Syntheses and optical properties of α-and β-Zn2SiO4:Mn nanoparticles by solvothermal method in ethylene glycol-water system. Mater. Chem. Phys. 120(2), 313–318 (2010)
M. Mai, C. Feldmann, Two-color emission of Zn2SiO4:Mn from ionic liquid mediated synthesis. Solid State Sci. 11(2), 528–532 (2009)
K. Omri, J. El Ghoul, A. Alyamani, C. Barthou, L. El Mir, Luminescence properties of green emission of SiO2/Zn2SiO4:Mn nanocomposite prepared by sol–gel method. Physica E 53, 48–54 (2013)
K. Omri, L. El Mir, Effect of manganese concentration on photoluminescence properties of Zn2SiO4:Mn nanophosphor material. Superlattices Microstruct. 70, 24–32 (2014)
B. Pandey, D.L. Weathers, Temperature dependent formation of ZnO and Zn2SiO4 nanoparticles by ion implantation and thermal annealing. Nucl. Instrum. Methods Phys. Res. Sect. B 332, 359–363 (2014)
B.C. Babu, B.V. Rao, M. Ravi, S. Babu, Structural, microstructural, optical, and dielectric properties of Mn2+: willemite Zn2SiO4 nanocomposites obtained by a sol–gel method. J. Mol. Struct. 1127, 6–14 (2017)
N.F. Samsudin, K.A. Matori, Z.A. Wahab, Y.W. Fen, J.Y.C. Liew, W.F. Lim, M.H.M. Zaid, N.A.S Omar, Manganese modified structural and optical properties of zinc soda lime silica glasses. Appl. Opt. 55(9), 2182–2187 (2016)
R. Ye, H. Ma, C. Zhang, Y. Gao, Y. Hua, D. Deng, P. Liu, S. Xu, Luminescence properties and energy transfer mechanism of Ce3+/Mn2+ co-doped transparent glass-ceramics containing β-Zn2SiO4 nano-crystals for white light emission. J. Alloys Compd. 566, 73–77 (2013)
M.K. Kretov, I.M. Iskandarova, B.V. Potapkin, A.V. Scherbinin, A.M. Srivastava, N.F. Stepanov, Simulation of structured 4T1→ 6A1 emission bands of Mn2+ impurity in Zn2SiO4: a first-principle methodology. J. Lumin. 132(8), 2143–2150 (2012)
K. Omri, O.M. Lemine, L. El Mir, Mn doped zinc silicate nanophosphor with bifunctionality of green-yellow emission and magnetic properties. Ceram. Int. 43(8), 6585–6591 (2017)
V. Sivakumar, A. Lakshmanan, S. Kalpana, R.S. Rani, R.S. Kumar, M.T. Jose, Low-temperature synthesis of Zn2SiO4:Mn green photoluminescence phosphor. J. Lumin. 132(8), 1917–1920 (2012)
A.J.A. Al-Nidawi, K.A. Matori, A. Zakaria, MHM Zaid (2017) Effect of MnO2 doped on physical, structure and optical properties of zinc silicate glasses from waste rice husk ash. Results Phys. 7(1), 955–961
M. Mazaheri, A.M. Zahedi, S.K. Sadrnezhaad, Two-step sintering of nanocrystalline ZnO compacts: effect of temperature on densification and grain growth. J. Am. Ceram. Soc. 91(1), 56–63 (2008)
Q.Y. Zhang, K. Pita, C.H. Kam, Sol–gel derived zinc silicate phosphor films for full-color display applications. J. Phys. Chem. Solids 64(2), 333–338 (2003)
C. Bertail, S. Maron, V. Buissette, T. Le Mercier, T. Gacoin, J.P. Boilot, Structural and photoluminescent properties of Zn2SiO4:Mn2+ nanoparticles prepared by a protected annealing process. Chem. Mater. 23(11), 2961–2967 (2011)
T.H. Cho, H.J. Chang, Preparation and characterizations of Zn2SiO4:Mn green phosphors. Ceram. Int. 29(6), 611–618 (2003)
M.H.M. Zaid, K.A. Matori, H.J. Quah, W.F. Lim, H.A.A. Sidek, M.K. Halimah, W.M.M. Yunus, Z.A. Wahab, Investigation on structural and optical properties of SLS–ZnO glasses prepared using a conventional melt quenching technique. J. Mater. Sci. 26(6), 3722–3729 (2015)
A. Tarafder, A.R. Molla, C. Dey, B. Karmakar, Thermal, structural, and enhanced photoluminescence properties of Eu3+-doped transparent willemite glass–ceramic nanocomposites. J. Am. Ceram. Soc. 96(8), 2424–2431 (2013)
A. Tarafder, A.R. Molla, S. Mukhopadhyay, B. Karmakar, Fabrication and enhanced photoluminescence properties of Sm3+-doped ZnO–Al2O3 –B2O3 –SiO2 glass derived willemite glass–ceramic nanocomposites. Opt. Mater. 36(9), 1463–1470 (2014)
N. Effendy, Z.A. Wahab, M.K. Halimah, K.A. Matori, H.A.A. Sidek, M.H.M. Zaid Structural and optical properties of Er3+-doped willemite glass-ceramics from waste materials. Opt. Int J Light Electron Opt. 127(24), 11698–11705 (2016)
J. El Ghoul, K. Omri, A. Alyamani, C. Barthou, L. El Mir, Synthesis and luminescence of SiO2/Zn2SiO4 and SiO2/Zn2SiO4:Mn composite with sol–gel methods. J. Lumin. 138, 218–222 (2013)
K.W. Park, H.S. Lim, S.W. Park, G. Deressa, J.S. Kim, Strong blue absorption of green Zn2SiO4:Mn2+ phosphor by doping heavy Mn2+ concentrations. Chem. Phys. Lett. 636, 141–145 (2015)
J. Liu, Y. Wang, X. Yu, J. Li, Enhanced photoluminescence properties of Zn2SiO4:Mn2+ co-activated with Y3+/Li+ under VUV excitation. J. Lumin. 130(11), 2171–2174 (2010)
Acknowledgements
The financial support from Ministry of Science, Technology and Innovation, Malaysia and Universiti Putra Malaysia (UPM), each under the Fundamental Research Grant Scheme (FRGS) and Research University Grant Scheme (RUGS) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaid, M.H.M., Matori, K.A., Aziz, S.H.A. et al. Enhanced luminescence properties of low-cost Mn2+ doped willemite based glass–ceramics as potential green phosphor materials. J Mater Sci: Mater Electron 28, 12282–12289 (2017). https://doi.org/10.1007/s10854-017-7045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7045-9