Abstract
The tungstenbronze-type-like Single-phase polycrystalline ceramics Ba6−3xSm8+2xTi18O54 (x = 0.5) doping with Ca ions had been prepared through the conventional solid-state reaction process. The phases and structure of the ceramics were analyzed through X-ray diffraction and scanning electron microscopy. The microwave dielectric properties of the ceramics were studied using a network analyzer. In this paper, the quality factor (Q × f) by substituting Ca ions for Ba ions in (Ba1−αCaα) Sm2Ti4O12 solid solutions in A1-sites was reported. In addition, the relationship between crystal structure and microwave dielectric properties was discussed from the viewpoint of structural formula and occupational state of large cations such as Ba, Ca ions in A1-sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microwave dielectric ceramic materials are rapidly developed in wireless communications, such as dielectric resonators in filters, phase shifters, and dielectric resonator antennae. Therefore, microwave dielectrics with high dielectric constant (εr) to enable device miniaturization, a low dielectric loss in the high-frequency region (i.e. high quality factor, Q × f) for good signal discrimination, and near-zero temperature coefficient (τf) of resonant frequency for temperature stability are of significant importance for these applications [1–4]. One of dielectric materials with high dielectric constant (εr) is tungstenbronze-type-like Ba6−3xR8+2xTi18O54 (R = rare earth) solid solutions. The crystal structure of tungstenbronze-type-like Ba6−3xR8+2xTi18O54 solid solutions was showed in these articles [5, 6].
Tungsten bronze-type like solid solutions with a formula of Ba6−3xR8+2xTi18O54 feature excellent microwave properties; thus they have been employed in mobile phones and other microwave devices. Bolton et al. found that the compositions from BaO–Sm2O3–TiO2 ternary system have good capacitance temperature stability; thus, they are suitable for temperature compensating ceramic capacitors [7]. Kolar et al. [8] and Nishigaki et al. [9] proposed BaO–Nd2O3–TiO2 and BaO–Sm2O3–TiO2 for ceramic filters due to their good dielectric properties.
The compounds BaO–Sm2O3–TiO2 were appreciably developed and attracted the interest of both research and industry because they featured good microwave properties, high permittivity, and a high quality factor respectively. However their large negative or positive temperature coefficients of the resonant frequency (τf) values are far beyond the requirement of the microwave devices [10–15].
Compositional modification, such as partial substitution or formation of a solid solution is suggested to optimize the dielectric properties of microwave dielectric ceramics.Imaeda et al. [16, 17] studied (Ba1−αSrα)6−3xSm8+2xTi18O54 solid solutions where x = 0.6 and 0 ≤ α ≤ 0.2. As according to this report, a quality factor (Q × f) of these solid solutions was improved by increasing lattice stability, which resulted from substituting Sr ions with smaller ionic radius for Ba ions. However the relative permittivity decreased sharply and the temperature coefficient of resonant frequency (τf) were still far above the 0 ppm/°C.
In this paper, the crystal structure of (Ba1−αCaα) Sm2Ti4O12 (BCST)solid solutions were demonstrated i.e. (Ba1−αCaα)6−3xSm8+2xTi18O54 where x = 0.5. Furthermore, microwave dielectric properties of (Ba1−αCaα)Sm2Ti4O12 solid solutions, and α = 0.01, 0.03, 0.05 and 0.07, were reported. Relationship between crystal structure and microwave dielectric properties was discussed from the viewpoint of structural formula and occupational state of large cations such as Ba, Ca ions in A1-sites.
2 Experimental procedure
(Ba1−αCaα) Sm2Ti4O12 ceramic samples with α = 0.01, 0.03, 0.05, 0.07 were fabricated by conventional solid-state reaction process from the starting materials including Sm2O3 (99.9%), CaCO3 (99.8%), BaCO3 (99.7%), TiO2 (99.9%).The raw materials were dried and weighed according to the above formalism and milled with ZrO2 balls in ethanol for 24 h. The wet mixed powders were dried and calcined at the temperature about 1000 °C for 2 h in an alumina crucible. The calcined powders were reground for 24 h, dried, mixed with 7 mol% PVA as binder and granulated. The granulated powders were uniaxially pressed into compacts with 15 mm in diameter and 6–8 mm in height under the pressure of 100 MPa. The compacts were sintered at optimum temperature for 4 h. In order to prevent the evaporation loss, the compacts were muffled with powder of the same composition. The cooling rate of 100 °C/h was executed till room temperature.
The phase constituents of the sintered samples were identified by X-ray powder diffraction (XRD) with Ni-filtered CuKa radiation (40 KV and 40 mA, Model advanced D8 Bruker). Bulk densities of the sintered specimens were identified by the Archimedes’ method. The microstructure of the sintered sample was characterized by scanning electron microscopy (SEM) (Model Hitachi S-4800, Hitachi Ltd., Japan). All samples were polished and thermally etched at the temperature which was 150 °C lower than its sintering temperature. Microwave dielectric properties of the sintered samples were measured about 3.6 GHz using a network analyzer(Advantest R3767C, Tokyo, Japan). The εr was measured according to the Hakki–Coleman method using the TE011 resonant mode, and the τf was measured using invar cavity in the temperature range from 20 to 80 °C.
3 Results and discussion
3.1 Crystalline structure and microstructure
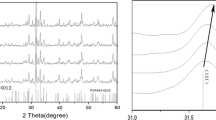
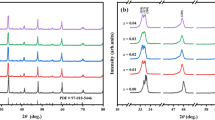
The (Ba1−αCaα) Sm2Ti4O12 ceramic with α = 0.01, 0.03, 0.05, 0.07 samples with faint yellow color, no cracks after sintering meant well sintered. Their linear shrinkage (i.e. from the green body to the sintered specimens) was approximately 15%. The X-ray diffractograms of the sintered BCST ceramics with different amounts of Ca ions addition (1–7 mol%) are plotted in Fig. 1a and the expanded graph in the region (2θ of 31.2°–32.2°) is presented in Fig. 1b. The end of sample’s name about BCST-1, BCST-3,BCST-5,BCST-7 means the (Ba1−αCaα) Sm2Ti4O12 with different amount of CaO addition (in mol%) of the samples.
The patterns of the samples matched well with the orthorhombic tungsten bronze structure of the BaSm2Ti4O12 samples (PDFNO.43–0235). The strongest diffraction peaks appeared at 2θ = 31.7° should be attributed to the (151) plans of BaSm2Ti4O12. No other characteristic peaks from other crystalline forms were detected, indicating that no obvious secondary phases were present.
Figure 1 showed all samples doped with different amounts of Ca ions have the same main diffraction patterns except the position of diffraction peaks little difference. It meant doping Ca ions did not have great influence on the BCST crystal structure till the content of the doped ca2+ increased to 3%. This was due to the solubility of CaO in the lattice without additional phase when the content of addition was small. As the Ca content (α) increased to 0.03 in BCST, the diffraction peaks of the BCST powders shifted towards to a lower angle, which indicated that the volume of unit cell increased and the results agreed well with Table 1. It was also worth noting that the full-width-at-half-maximum(FWHM) of the diffraction peak varies remarkably with the Ca content, which should be correlated with the magnitude of grain size.
Figure 2 demonstrates microstructures of thermally etched BCST (α = 0.01, 0.03, 0.05 and 0.07) ceramics after sintering at their optimal sintering temperatures. Dense and homogenous microstructures were observed in all samples. No apparent grains of a secondary phase were observed in the Ca ions doped BCST ceramics. The results matched X-ray analysis (Fig. 1a) fairly well.
The grains are mostly typical hexagonal grain morphology sized for undoped BaSm2Ti4O12. With increasing CaO content the difference between the larger grains and the smaller grains became more pronounced and irregular in shape Fig. 2c, d. Obviously, addition of small amounts of dopants i.e. 0.01 and 0.03 mol CaO enhances the grain size. A small amount of porosity can be observed at α = 0.03. Moreover, the general grain size of ceramics with α = 0.07 was much smaller than the others, which is in good accordance with the variation of FWHM shown in Fig. 1b.
Generally, with increasing CaO doping level, an enlarged grain size should be expected as more liquid phase would form to promote the grain growth [18]. Nevertheless, rare earth oxide often plays a role of grain growth inhibitor in many ceramics [19], which should be responsible for the unexpected decline of grain size in Fig. 2(c, d).
3.2 Densification
The influence of the amount of Ca ions added on the values of the relative density of the sintered BCST ceramics was plotted in the curve of Fig. 3. In BCST ceramics, the relative density decreased with α increase as a small amount of porosity can be observed. However when α up to 7% the densification increased to 94.7% of the theoretical density.
3.3 Microwave dielectric properties
The Q × f values and dielectric constant of BCST ceramics with different amounts of Ca ions sintered at their optimal sintering temperatures for 4 h were shown in Fig. 4.
The Q × f of undoped BaSm2Ti4O12 ceramics is about 10,000 GHz [11]. The Q × f of BCST ceramics decreased obviously when α up to 3%, meanwhile the εr varied slightly according with the relative density (seen from Fig. 3).
Q × f value is generally affected by crystallizability, oxygen vacancy, long-range ordering degree of cations, inner stress of the crystals and phase constitution. In the present situation, Ca substitution for Ba leaded to alter the crystal cell volume (seen from Table1) and decrease the reduced mass which increases the Q × f value according the following equation:
where V is the crystal cell volume in Å3, γ is damping constants, m is reduced mass, Z is charge valences; However the γ was greatly influenced by grain boundaries、porosity, so decline of grain size increased the grain boundaries and the amount of porosity can decreased Q × f value greatly.
The Clausius–Mosotti equation shows explicitly how dielectric constant depends on composition and crystal structure through polarizability and molar volume as following:
where V is the crystal cell volume in Å3, χ is the cell polarization. The polarizability of Ca2+ 3.16 Å3 is lower than that of Ba2+(6.40 Å3) [20, 21] and consequently the total polarizabilities reduced significantly with increasing α due to the substitution of Ca2+ for Ba2+, which leads to the decrease of εr. Although the increase of crystal cell volume resulted in the increase of εr; but it was relatively not so much. On the other hand, the influence of relative density on the dielectric constant was more significant. Therefore, there was a decrease of εr with α which matched well with the relative density.
The temperature coefficient of the resonant frequency τf correlated with the structural and compositional characteristics of a material.
From Fig. 5, the BCST ceramics approached to zero value τf with 1.0 mol% Ca ions additions. The value of τf can be adjusted by Ca ions additions with changing the tolerance factor (t). The tolerance factor can be calculated by the following equation:
where R is the ionic radius in Å, in the (Ba1−αCaα)6−3xSm8+2xTi18O54 systems (in this paper x = 0.5). This structural formula can be represented as [Ba0.5−αCaαSm9V0.5]A1[Ba4]A2Ti18O54 [22]. Some Ba ions in A1-sites are substituted by Ca ions. As a result, the t decreased, the τf move toward to the positive value. So the τf value increases almost linearly and changes from negative to positive in the region of 0.01 ≤ α ≤ 0.07.
4 Conclusion
Single-phase ceramics can be prepared by the Ca substitution of BaSm2Ti4O12, resulting in the formation of (Ba1−αCaα) Sm2Ti4O12 based solid solutions for determined sintering conditions. The addition of Ca ions, tested up to 7 mol%, did not alter the main orthorhombic tungsten bronze structure of the crystalline phase. The values of εr and τf can be influenced by varying the amount Ca ions. The values of Q × f exhibited a non-monotonous behavior and deteriorated greatly with the increase of Ca ions addition. For the BCST ceramics, the best values of the dielectric properties were obtained with 1.0 mol% Ca ions addition as εr = 76.1, Q × f = 9000 GHz, and τf = −1.3 ppm/°C.
References
R. Freer, F. Azough, Microstructural engineering of microwave dielectric ceramics. J. Eur. Ceram. Soc. 28, 1433–1441 (2008)
E.A. Nenasheva, N.F. Kartenko, High dielectric constant microwave ceramics. J. Am.Ceram. Soc. 21, 2697–2701 (2001)
I.M. Reaney, D. Iddles, Microwave dielectric ceramics for resonators and filters in mobile phone networks. J. Am. Ceram. Soc. 89, 2063–2072 (2006)
M.T. Sebastian, Dielectric materials for wireless communication. first ed. Elseiver, Oxford (2008)
H. Ohsato, Science of tungstenbronze-type like Ba6–3xR8+2xTi18O54(R = rare earth) microwave dielectric solid solutions. J. Eur. Ceram. Soc. 21, 2703–2711 (2001)
M. Valant, D. Suvorov, C.J. Rawn, Intrinsic reasons for variations in dielectric properties of Ba6–3xR8+2xTi18O54(R = La-Gd) solid solutions. Jpn. J. Appl. Phys. 38(5A), 2820–2826 (1999)
R.L. Bolton, Temperature compensating ceramic capacitors in the system baria rare earth oxide titania. PhD thesis. Ceramic Engineering, University of Illinois, Urbana (1968)
D. Kolar, Z. Stadler, S. Gaberscek, D. Suvorov, Ceramic and dielectric properties of selected compositions in the BaO-TiO2-Nd2O3 system. Ber. Dt. Keram. Ges. 55, 346–347 (1978)
S. Nishigaki, H. Kato, S. Yano, R. Kamimura, Microwave dielectric properties of (Ba,Sr)O–Sm2O3–TiO2 ceramics. J. Am. Ceram. Soc. Bull. 66, 1405–1410 (1987)
H. Ohsato, M. Mizuta, T. Ikoma, Z. Onogi, S. Nishigaki, T. Okuda, Microwave dielectric properties of tungsten bronzetype Ba6–3xR8+2xTi18O54 (R = La, Pr, Nd and Sm) solid solutions. J. Ceram. Soc. Jpn. 106(2), 178–182 (1998)
J. Takahashi, T. Ikegami, K. Kageyama, Occurrence of dielectric 1:1:4 compound in the ternary system BaO–Ln2O3–TiO2 (Ln = La, Nd, and Sm):II.Reexamination of formation of isostructural ternary compounds in identical systems. J. Am. Ceram. Soc. 74(8), 1873–1879 (1991)
H. Okudera, M. Nakamura, H. Toraya, H. Ohsato, Tungsten bronze-type solid solutions Ba6–3xR8+2xTi18O54 (x = 0. 3, 0.5,0. 67, 0.71) with superstructure. J. Solid State Chem. 142, 336–343 (1999)
H. Ohsato, H. Kato, M. Mizuta, S. Nishigaki, T. Okuda, Microwave dielectric properties of the Ba6–3x(Sm1–yRy)8+2xTi18O54 (R = Nd, La) solid solutions with zero temperature coefficient of the resonant frequency. Jpn. J. Appl. Phys. 34, 5413–5417 (1995)
H. Ohsato, T. Ohhashi, S. Nishigaki, T. Okuda, K. Sumiya, S. Suzuki, Formation of solid solution of new tungsten bronze-type microwave dielectric compounds Ba6–3xR8+2xTi18O54 (R = Nd and Sm, 0 ≤ x ≤ 1). J. Appl. Phys. 32, 4323–4326 (1993)
R. Ubic, I.M. Reaney, W.E. Lee, Microwave dielectric solid-solution phase in system BaO-Ln2O3-TiO2(Ln = Lanthanide caution). Int. Mater. Rev. 43, 205–219 (1998)
M. Imaeda, K. Ito, M. Mizuta, H. Ohsato, S. Nishigaki, T. Okuda, Microwave dielectric properties of Ba6–3xSm 8+2 x Ti18O54 solid solutions with Sr substituted for Ba. Jpn. J. Appl. Phys. 36, 012 (1997)
I. Kagomiya, M. Suzuki, K. Kakimoto, H. Ohsato, Microwave dielectric properties of tungsten bronze type like (Ba1–αSrα)6–3xR8+2xTi18O54 (R = Sm, Nd) solid solutions. J. Eur. Ceram. Soc. 27, 3059–3062 (2007)
R. Lowndes, F. Azough, R. Cernik, R. Freer, Structures and microwave dielectric properties of Ca(1–x)Nd2x/3TiO3 ceramics. J. Eur. Ceram. Soc. 32(14), 3791–3799 (2012)
W.Q. Luo, Z.Y. Shen, Y.M. Li, Z.M. Wang, R.H. Liao, X.Y. Gu, Structural characterizations, dielectric properties and impedance spectroscopy analysis of NdxSr1–1.5xTiO3 ceramics. J. Electroceram. 31(1–2), 117–123 (2013)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Crystallogr. Section A. 32(5), 751–767 (1976)
R.D. Shannon, Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phy. 73(1), 348–366 (1993)
M. Suzuki, H. Ohsato, K. Kakimoto, T. Nagatomo, T. Otagiri, Crystal structure and microwave dielectric properties of (Ba1−αSrα)6−3xSm8+2xTi18O54 solid solutions. J. Eur. Ceram. Soc. 26(10), 2035–2038 (2006)
Acknowledgements
The project was supported by the National Natural Science Foundation of China (No. 51402251).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Luo, J., Guan, H. et al. Effects of CaO additives on the structure and properties of Ba6−3xSm8+2xTi18O54 (x = 0.5) ceramics. J Mater Sci: Mater Electron 28, 10338–10342 (2017). https://doi.org/10.1007/s10854-017-6802-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6802-0