Abstract
A novel flexible photoanode based on a silver nanowire (AgNW)/polymer composite electrode was fabricated and used for dye-sensitized solar cells. The AgNW/polymer composite substrate comprised a thin percolation network of AgNWs embedded on the surface of polyacrylic ester. As titanium dioxide film formed on top of the composite substrate, the effect of compression was investigated. Drop-cast sensitization was then used for both pressed and nonpressed photoanode, and the nonpressed one performed better. A cell efficiency of 0.91% was achieved under 100 mW cm−2 simulated solar irradiation. After a bending test on the flexible photoanode, the solar cell retained 0.71% efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In 1991, Michael Grätzel and coworkers first reported dye-sensitized solar cells (DSSCs) based on a thin layer of nanocrystalline TiO2 coated with a monolayer of ruthenium-based dye to sensitize the film for light harvesting [1]. Since then, DSSCs have been considered as third-generation solar cells and intensively studied over the past two decades [2–5]. A typical DSSC consists of three components: a photoanode (i.e., a working electrode), a counter electrode, and an electrolyte (usually an I−/I −3 redox couple) sandwiched between these two electrodes. The photoanode is usually a dye-molecule-coated nanocrystalline porous TiO2 film deposited onto a transparent conductive glass substrate, and the most commonly used counter electrode is a platinum-coated conductive substrate [6–8]. Flexible DSSCs (or DSSCs with one flexible electrode) based on polymer substrate are attracting considerable attention because of their unique characteristics, such as ease of fabrication and mass production, low cost, light weight, and flexible nature [4–6]. Replacing rigid glass conductive substrates with flexible counterparts in DSSCs reduces weight and cost, as well as extends DSSC applications, such as in constructing different shapes of building surfaces and in mobile power sources for portable electronic devices [6, 9, 10]. Conventional TiO2 photoanodes are sintered at a high temperature (400–550 °C) to remove organic additives and to promote chemical bonding among particles to establish their electrical connection [1, 11]. However, high-temperature treatment is not applicable to flexible plastic substrates. To date, many efforts have been exerted toward the low-temperature preparation of TiO2 films in DSSCs, such as mechanical compression [12, 13], chemical sintering of TiO2 colloid solution [14, 15], and microwave irradiation of TiO2 films [16]. Among these the compression method achieves the highest cell efficiency [13, 17].
The most commonly used conductive plastic substrates are polyethylene naphthalate (PEN) or polyethylene terephthalate (PET) coated by transparent indium tin oxide (ITO). ITO/PET and ITO/PEN substrates are expensive to produce, and the element indium in these substrates harms solar cells and the environment.
In this paper, we propose the use of a novel AgNW/polymer conducting substrate for the flexible photoanode of DSSCs. This AgNW/polymer composite substrate comprises a thin percolation network of silver nanowires inlaid in the surface of the polymer, such as polyacrylic ester. This substrate has been applied to polymer light-emitting devices and organic thin-film transistors [18, 19]. Besides the high transparency and light weight, the substrate holds other advantages over ITO/PET and ITO/PEN substrates. Such benefits include low cost, simple production, and environment friendliness. For example, the fabrication of ITO film needs valuable equipment, and the preparation process (typically sputtering) demands stringent specification and high energy consumption. For the composite substrate, AgNW film could be prepared by screen printing or spraying in mass production to greatly reduce the cost. What’s more, the network of AgNWs is embedded on the surface of polyacrylic ester, so AgNW and polymer is tightly bonded. Accordingly, we further fabricated and characterized DSSCs based on this novel substrate and conducted a bending test on the flexible photoanode.
2 Experimental
2.1 Materials
A series of acrylate monomer could be used for fabricating the composite [18, 19]. Here we choose ethoxylated (4) bisphenol a dimethacrylate (provided by Eternal Materials Co., Ltd., Taiwan) since it has the virtue of colorless and transparent, and the corresponding polymer is flexible and adjustable. 2, 2-Dimethoxy-2-phenyl-acetophenone (DMPA) was purchased from Sigma–Aldrich. AgNWs were synthesized with an average diameter of 25–35 nm and average length of 10–20 μm [18, 20–22]. N719 ruthenium dye, LiI, I2, 1-propyl-3-methylimidazolium iodide, 4-tert-butylpyridine, TiO2 paste, and fluorine-doped tin oxide (FTO) conducting glass were purchased from Yinkou OPV Tech New Energy Co., Ltd. (Yinkou, China). Sodium citrate and silver nitrate were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were reagent grade and used as received without further purification.
2.2 Preparation of silver composite substrate
The dispersion of AgNWs in isopropanol with a concentration of 2 mg/ml was coated on precleaned glass substrates using a Meyer rod (RD Specialist) [18, 20–22]. To enhance the contact of touching AgNWs, silver nanoparticles (Ag NPs) were synthesized on top of the as-prepared AgNW film [20–22]. First, 1% aqueous solution of sodium citrate and silver nitrate solution of 0.1 M were prepared, and then an adequate amount of silver nitrate solution was heated to boiling. The glass substrates with AgNW film on top were immersed in the solution. Sodium citrate solution was added, and the mixture was allowed to boil for a time period under white light. Afterwards, acrylate monomer with 1 wt% DMPA as polymerization initiator was coated on the AgNW coating. The coatings were then cured under an ultraviolet curing conveyor for about 90 s and peeled off as a transparent flexible composite electrode.
2.3 Preparation of TiO2 film
The commercial TiO2 viscous paste used was composed of TiO2 nanoparticles (Degussa P25), ethyl cellulose, terpineol, and ethanol. The TiO2 paste was deposited onto the composite conducting substrate by screen printing.
The as-prepared film was treated by two methods to form a dense oxide semiconductor layer.
2.3.1 Method 1
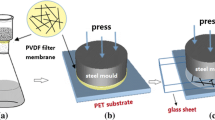
As reported by D. Zhang et al. and H. Lindström et al. [23, 24], porous nanostructured TiO2 film can be formed by compressing a TiO2 particle layer on the conducting plastic substrate. The TiO2 film on the composite conducting substrate was dried at room temperature and placed between smooth steel sheets, and then about 35 MPa pressure was applied to the sheets by using a punching machine.
2.3.2 Method 2
No pressure was applied and the TiO2 film was left to air dry. If the ambient temperature is lower than 20 °C, the film would be slightly heated at about 50 °C on a hot plate (Thermo Scientific Cimarec).
2.4 Sensitization of photoanode
A 0.5 mM ethanol solution of Ru complex dye N719 was utilized to sensitize the TiO2 film at room temperature. Dipping TiO2 film in the sensitizer solution is the most commonly used technique; however, TiO2 films prepared without sintering treatment easily break off upon dipping or afterwards. To produce high-integrity sensitized TiO2 film, a drop-cast method was applied with a self-designed device. N719 solution was very slowly added dropwise onto TiO2 film, with the sensitizing time usually proceeding for about 2 days. The sensitized TiO2 film then air dried.
2.5 Assembly of DSSCs
The sensitized TiO2 films were used as working electrodes, i.e., photoanodes, with a 100 nm Pt-deposited FTO glass as counter electrode. The electrolyte, which consisted of 0.3 M LiI, 0.6 M 1-propyl-3-methylimidazolium iodide, 0.05 M I2, and 0.5 M 4-tert-butylpyridine in acetonitrile, was injected between the photoanode and counter electrode through a siphonic effect. The active area of the cells was 0.16 cm2.
2.6 DSSC characterization
The photovoltaic performances (short circuit current, open circuit voltage, fill factor, and power conversion efficiency) of the cell were examined by measuring the current–voltage (I–V) characteristics of the cells using a Keithley 2450 source meter under a light intensity of 100 m Wcm−2 offered by a xenon lamp (300 W; Nbet, HSX-F300). The microstructure of AgNW films was analyzed with a field-emission scanning electronic microscope (JEOL and JSM7100F).
3 Results and discussion
Figure 1a, b show the scanning electronic microscopy (SEM) images of the surfaces of the flexible AgNW/polymer composite electrode without or with Ag NPs on top, respectively. The AgNWs on the flexible substrate were disorderly arranged. With the addition of Ag NPs, the nodes where the two silver nanowires joined became tightened joints. This development benefitted the electroconductivity of AgNWs. The link among AgNWs was further stabilized by these enhanced joints. Furthermore, the electrical conductivity of AgNW/polymer composite substrate was measured by a four probe meter (RTS-9, 4Probes Tech, China). The sheet resistance of the composite substrate containing only AgNWs is 15 Ωsq−1, while the sheet resistance of the substrate containing both AgNWs and Ag NPs is 11 Ωsq−1. The introduction of Ag NPs into AgNW/polymer composite improves the conductivity.
The transmittance of the flexible AgNW/polymer composite electrode is shown in Fig. 2, and the transmission of FTO conducting glass and the commonly used flexible substrate PEN/ITO is displayed. The novel AgNW/polymer composite electrode had better transparency than electrodes with FTO glass and PEN/ITO substrate.
The plot of photocurrent density (J) versus photovoltage (V) of flexible DSSCs based on AgNW/polymer composite electrode is presented in Fig. 3, and the detailed photovoltaic properties, i.e. short-circuit current density (J SC ), open circuit voltage (V OC ), fill factor (FF), and efficiency (η) are also described in Table 1. Under illumination of one sun (100 mW/cm2), J sc of 1.71 mA/cm2 was obtained by novel flexible DSSC with TiO2 film prepared by compression (method 1). And the energy conversion efficiency was 0.78%. The nonpressed device exhibits higher J sc of 5.58 mA/cm2 which leads to a higher energy conversion efficiency of 0.91%. The open circuit voltages of DSSCs prepared with two methods are very close (0.55 V and 0.54 V, respectively). The overall series resistance, which can be approximated as the slope of the J–V curves at far forward bias, is nominally the same for both devices [25]. However, it should be noted that, for solar cells with TiO2 film prepared by compression (method 1), there is a “peak” in the J–V curve around bias of 0.3 ~ 0.4 V. What’s more, the FF of these devices is high (0.826). A low J sc value leads to a high FF unrealistically, since FF is obtained by a division of the maximum output electric power P max by the production of V oc and photocurrent (I sc ) [7, 8]. The reason might be unsuccessful assembling of the solar cells or inefficient device structure. Since the “peak” in the J–V curve occurs for devices prepared with method 1 in all batches, it is probably that the compression has adverse effect on the composite substrate based photoanode, and the press technique might be unsuited to flexible DSSCs based on novel AgNW/polymer composite. This situation does not occur for the solar cell with TiO2 film prepared without compression (method 2) and the devices have modest FF of 0.301.
SEM images of pressed and nonpressed TiO2 film upon the composite conducting substrate were obtained to further explore the effect of compression on the photoelectrode. As shown in Fig. 4a, the surface of pressed TiO2 film was flattened, and the film was compact and homogeneous. However, for nonpressed TiO2 film (Fig. 4b), the surface was fluctuant and rough, and the film is relatively loose and porous. A dense and tightly connected TiO2 structure is necessary to operate traditional DSSC; however, the situation here differed for TiO2 not subjected to high-temperature sintering. For TiO2 deposited onto the composite substrate, the nonpressed sample better absorbed the electrolyte. We observed that the electrolyte took more time to permeate through the pressed sample than through the nonpressed one. Figure 4c shows the cross-section SEM image of the nonpressed polymer/AgNW/TiO2 electrode. As seen, AgNW is well embedded on the surface of polymer and in good contact with TiO2 film.
Furthermore, DSSCs based on nonpressed TiO2 film easily maintains their integrity during fabrication and testing. Figure 5 shows the pictures of the photoanodes. Figure 5a–c show the photos of nonpressed photoanode after dye sensitization, electrolyte permeation, and photovoltaic testing, respectively. Figure 5d–f show those of pressed photoanode after dye sensitization, electrolyte permeation, and photovoltaic testing, respectively. The nonpressed photoanode had slight damage even after photovoltaic characterization. However, the breakage on the pressed photoanode was serious and blocks of TiO2 fell of the substrate. This breakage increased in severity as fabrication proceeded, especially after photovoltaic testing. The contact with liquid easily resulted in damage on the pressed TiO2 film. However, the as-prepared nonpressed photoanode held together during fabrication and characterization even when in contact with liquid.
The novel flexible photoanode (prepared without mechanical compression) was subjected to a bending test to evaluate its flexibility. After sensitization, the flexible photoanode was bended along a 27 mm-diameter cylinder, and the bending angle of the photoanode was calculated to be 17°. After 500 times of repeated bending, some decrease in cell performance was observed. Photovoltaic parameters also slightly decreased, although cell efficiency was acceptable at 0.71%. The open-circuit voltage, short-circuit photocurrent density, and FF were 0.58 V, 2.91 mA/cm2 and 0.417, respectively. And its J–V curve is shown as the inset of Fig. 3. This result may be due to the fact that the test led to battery loss, or that the linkage among TiO2 nanoparticles did not appear fairly close after bending.
As H. Lindström, et al. and T. Yamaguchi, et al. reported, flexible DSSCs based on ITO/PET and ITO/PEN systems achieve cell efficiency as high as nearly 8% by press technique [12, 13]. Herein, the effect of compression was investigated and the sample prepared without pressing exhibits better performance, and a drop-cast sensitization was used instead of traditional dipping sensitization. Further performance optimization of these flexible DSSCs based on novel conducting composite substrate is under study in our group, as well as comparative study of different flexible substrates.
4 Conclusions
We fabricated and investigated a novel flexible photoanode based on AgNW/polymer composite and the corresponding DSSCs. The effect of compression on the photoelectrode was studied, and a slow drop-cast sensitization was carried out. The solar energy conversion efficiency of DSSCs not subjected to compression was 0.91% under 100 mW cm−2, and the counterpart device subjected to compression had a lower efficiency of 0.78%. The flexibility of the novel photoanode was further investigated through a bending test.
References
B. O’Regan, M. Gratzel, Nature 353, 737 (1991)
M. Grätzel, Chem. Lett. 34, 8 (2005)
Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide, L. Han, Jpn. J. Appl. Phys. 45, 24 (2006)
P. N. Didwal, K. S. Pawar, P. R. Chikate, A. C. Abhyankar, H. M. Pathan, R. S. Devan, J. Mater. Sci. 27, 12446 (2016)
F. Deng, X. Mei, X. Wan, R. Fan, Q. Wu, X. Yan, L. Wan, D. Shi, Y. Xiong, J. Mater. Sci. 26, 7635 (2015)
H.C. Weerasinghe, F. Huang, Y.B. Cheng, Nano Energy 2, 174 (2013)
P. Wang, S.M. Zakeeruddin, P. Comte, R. Charvet, R.H. Baker, M. Grätzel, J. Phys. Chem. B 107, 14336 (2003)
S. Ito, T.N. Murakami, P. Comte, P. Liska, C. Grätzel, M.K. Nazeeruddin, M. Grätzel, Thin solid films 516, 4613 (2008)
L. Cao, C. Gong, J. Yang, Electrochim. Acta 192, 422 (2016)
K.R. Reddy, K.V. Karthikb, S.B.B. Prasadc, S.K. Sonid, H.M. Jeonge, A.V. Raghu, Polyhedron 120, 169 (2016)
C.J. Barbé, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover, M. Grätzel, J. Am. Ceram. Soc. 80, 3157 (1997)
H. Lindström, A. Holmberg, E. Magnusson, S.E. Lindquist, L. Malmqvist, A. Hagfeldt, Nano Lett. 1, 79 (2001)
T. Yamaguchi, N. Tobe, D. Matsumoto, T. Nagai, H. Arakawa, Sol. Energy. Mater. Sol. Cells, 94, 812 (2010)
N.G. Park, K.M. Kim, M.G. Kang, K.S. Ryu, S.H. Chang, Y.J. Shin, Adv. Mater. 17, 2349 (2005)
H.C. Weerasinghe, G.V. Franks, J.D. Plessis, G.P. Simon, Y.B. Cheng, J. Mater. Chem. 20, 9954 (2010)
S. Uchida, M. Tomiha, H. Takizawa, M. Kawaraya, J. Photochem. Photobiol. A 164, 93 (2004)
K. Fan, R. Li, J. Chen, W. Shi, T. Peng, Sci. Adv. Mater. 5, 1947 (2013)
J. Liang, L. Li, X. Niu, Z. Yu, Q. Pei, Nat. Photon. 7, 817 (2013)
J. Liang, L. Li, D. Chen, T. Hajagos, R. Zhi, S.Y. Chou, W. Hu, Q. Pei, Nat. Commun. 6, 7647 (2016)
H. Lu, D. Zhang, J. Cheng, J. Liu, J. Mao, W.C.H. Choy, Adv. Funct. Mater. 25, 4211 (2015)
Z.S. Pillai, P.V. Kamat, J. Phys. Chem. B 108, 945 (2004)
S. Cumberland, J. Chromatogr. A 1216, 9099 (2009)
D. Zhang, T. Yoshida, K. Furuta, H. Minoura, J Photochem. Photobiol. A 164, 159 (2004)
H. Lindström, A. Holmberg, E. Magnusson, L. Malmqvist, A. Hagfeldt, J. Photochem. Photobiol. A 145, 107 (2001)
B.E. Hardin, W. Gaynor, I.K. Ding, S.B. Rim, P. Peumans, M.D. McGehee, Org. Electron 12, 875 (2011)
Acknowledgements
The research was supported by the Grants no. 61106127 and no. 51472034 from the National Natural Science Foundation of China, No. 2013CFA088 from the Science Foundation of Educational Commission of Hubei Province of China, No. 2015D-5006-0404 from Petro China Innovation Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, R., Zhang, C., Yin, X. et al. Novel flexible photoanode based on Ag nanowire/polymer composite electrode. J Mater Sci: Mater Electron 28, 10092–10097 (2017). https://doi.org/10.1007/s10854-017-6770-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6770-4