Abstract
Glass of the composition (mol%) (25-x) Na2O−xFe2O3−25PbO−50SiO2, x = 0, 0.25, 0.5, 1, 3, 5, 7, 10, 15, 16 and 20 (mol%) were prepared by the melt quenching method. The effect of replacing Fe2O3 for Na2O on the optical, structural, electrical and dielectric properties was studied. Experimental and theoretical density and XRD of the samples were also reported. Pb2+, Fe2+ and Fe3+ ions contributed to the UV-cut off and the developed bands in the visible and near IR regions. FTIR revealed the decrease of the SiO4 and increase of FeO4 units. XRD indicated change in the glass structure above 15 mol% Fe2O3. The electrical conduction seems to occur by ionic and electronic controlled electron hopping between Fe2+ and Fe3+ sites. The activation energy of ionic conduction decreased from 1.74 eV for un-replaced Na2O sample to 0.16 eV for sample with 16 mol% Fe2O3 at 1 MHz. Samples with 16 and 20 (mol% Fe2O3) showed ε′ values in the range from 115 to 186 and from 34 to 66, respectively. These results present promising candidates for the studied glasses to be used as energy storage materials in electronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lead silicate glasses are applied in many different areas such as video-screen manufacturing, electrode glass production, ophthalmic, biotechnology and radiation protection [1]. Glasses containing Fe2O3 are also used in electrochemical, electronic and electro-optic devices [2]. These glasses have very interesting structure and offer properties such as high value of the refractive index, low absorption in the visible region of the spectrum, an improved chemical stability and brightness [3, 4].
Lead oxide (PbO) is unique in its influence on the structure of glasses. Such interesting properties of these glasses are mainly due to the high polarizability and the relatively low field strengths of Pb cations as compared to other conventional glass formers such as SiO2 and GeO2 [5]. In lead silicate glasses, Pb2+ ions act as glass formers when PbO is present in high quantities and as network modifiers when lead oxide is present in lower concentrations [6]. When the structural role of Pb2+ is changed a considerable modification in the local environment of the silicate network occurs. It was suggested [7] that SiO2 is the main glass former in the lead silicate glasses when PbO is present up to 40% where PbO is mainly present as glass modifier. When PbO is present over and above this concentration the polymeric chains of PbO4 pyramids were found to be connected through silicate tetrahedra in the glass. However, it is reported [8] that, the PbO4 groups are dominating for any concentration of PbO and at low concentrations the co-existence of the PbO4 groups and PbO3 pyramids is possible.
Recent works have shown that principal features for Fe-containing glasses are dependent on the valence states of iron ions and their coordination in the glass network [9, 10]. When iron oxides are introduced into glasses, iron ions exist as equilibrium between the yellow ferric ion, Fe3+, and the blue ferrous ion, Fe2+ [11]. Most of ferrous iron is believed to exist in octahedral coordination as a network modifier [12]. On the other hand, ferric iron may exist in four fold tetrahedral, and/or six fold octahedral coordination [13–16]. The oxidation states of iron were extensively studied in silicate glasses and melts [17–19].
Limited ternary systems of silicate glasses containing Fe2O3 are investigated. Iron doped sodium lead borate glasses were studied by electrical measurements and Mössbauer spectroscopy [20]. Cerium lead borate glasses doped with iron oxide [21] and lithium lead borate glasses doped with silver oxide [22] were investigated. Electron para-magnetic resonance and optical study was performed for alkali lead tetra borate glasses doped with iron oxide [23]. The transport properties of Fe2O3–PbO–B2O3 glasses were studied by Sanjay et al. [5]. The optical absorption properties of lead silicate glasses containing high concentrations of iron oxide were studied by Ookawa et al. [24]. The electronic conductivity, XRD and atomic force microscopy and magnetic force microscopy in the SiO2–PbO–Fe2O3 glass were investigated [25]. Lead oxyfluoro silicate glasses containing iron ions were subjected to optical absorption, ESR, FTIR spectral investigations [6]. The role of Fe in a series of Fe2O3–PbO–SiO2–Na2O glasses in occupying octahedral or tetrahedral sites is investigated [26]. The dielectric constant of the system xFe2O3⋅(1−x)PbO⋅SiO2 in addition to IR and Raman spectra have been reported [27].
In previous studies on effect of replacing Fe2O3 for Na2O on the chemical durability and glass structure of glass of composition (25−x) Na2O−x–Fe2O3–25PbO–50SiO2 has been investigated [28]. The aim of the present work is to extend such investigation to investigate the electrical and dielectric properties which have been noticed to receive less attention. The optical and physical properties will also be correlated with the glass structures of glasses.
2 Materials and methods
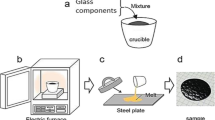
Glass samples of the composition (mol %) (25−x) Na2O−xFe2O3–25PbO–50SiO2 were prepared by the melt quenching method. Samples with x = 0, 0.25, 0.5, 1, 3, 5, 7, 10, 15, 16, and 20, were denoted as F0, F0.25, F0.5, F1, F3, F5, F7, F10, F15, F16, and F20, respectively. Table 1 lists the nominal composition of the studied glass samples. Chemically pure Na2CO3, Fe2O3 and Pb3O4 and washed silica sand were used as materials for glass batch preparation. The batch materials in the appropriate amounts were thoroughly mixed and melted in porcelain crucibles at a temperature of 1200 °C, using electrically heated furnace. Melting was continued under normal atmospheric conditions for 2 h after the last addition of the batch constituents. The melts were swirled about for every 30 min to ensure homogeneity and the melts were cast in rectangular forms, and samples were immediately transferred to electrically heated furnace at 450 °C. The furnace was switched off and left to cool to room temperature. Some of the obtained samples were transparent while others were opaque.
Optical absorption measurements were carried out on the transparent polished rectangular samples of dimensions 3 × 10 × 30 mm using Jasco V-530 spectrophotometer in the range 190–1100 nm.
FTIR measurements were carried out on powdered samples after being mixed with KBr and pressed into discs. FTIR-6100 Jasco instrument was used for measuring the transmittance in the range 400–4000 wave number cm−1.
X-ray powder diffraction (Bruker Axs, D8-Advance - X-ray Diffractometer, Germany) with Cu-target Cu Kα radiation with λ = 1.5405 Å, was used to identify the nature of the samples.
Archimedes method was used for density measurement using xylene of density (0.86 gcm− 3) as the immersing liquid. The density was calculated according using the equation: D = (W air / W air − W xylene) × ρxylene, where Wair and Wxylene are the weight of the glass sample in air and in xylene, respectively, and ρ is the density of xylene.
The electrical measurements were performed on the prepared samples after painting their opposite sides with silver paste. Measurements were carried out using a programmable automatic LCR HiTester (HIOKI, 3532-50), Japan over a frequency range from 0.042 kHz to 1 MHz and temperature range from 25 to 300 °C. The temperature was determined using a copper/Constantan thermocouple in close proximity to the sample. The dielectric constant (ε′), the loss tangent (tan δ), and the ac conductivity (σac) were determined based on the following expressions [29, 30]:
ε′ = C d / ε o A, ε′′ = ε′ tan δ, σ ac = ω ε o ε′′, where C = measured capacitance of the sample (F), d = thickness of the sample (m), ε o = permittivity of free space equals 8.85 × 10 −12 F m −1, A = Sample surface area (m2), ω = the angular frequency, tan δ = the loss tangent which is obtained directly from the instrument.
3 Results and discussion
3.1 Optical studies
Figure 1 shows the absorption spectra of the transparent glass samples F0, F0.25, F0.5, F1 and F3. Samples with higher content than 3 mol% Fe2O3 were opaque (blackish-brown) that makes absorption measurement very difficult (samples thickness 3 mm). From Fig. 1 it can be noticed that the base glass F0 has UV-cut off at 339 nm, which is associated with s2 lone pair electrons (s2 → s1p1) in Pb2+, and no absorption bands in the visible region. Upon the addition of Fe2O3 and increasing its content at the expense of Na2O the UV-cut off shifts to higher wavelengths. This shift can be ascribed to the O2−2p–Fe3+3d charge transfer in octahedral coordinated Fe3+ ions [13], which increases as the Fe2O3 content replacing Na2O increases. Charge transferring can also associate with adjacent redox species of iron (Fe2+–O2−–Fe3+) where the relevant electronic orbital involves both the ferrous to ferric species. It is reported that, both ferrous and ferric ions absorb UV light below 350 nm due to the charge transfer process [31, 32].
The spectra of glasses F0.25 and F0.5 show bands at 379 nm (obscured in F0.5 due to the shift of its UV cut off), 402 and 445 nm, due to d-d transitions of Fe3+ ions in FeO6 units [33]. These bands cannot be observed in the spectra of other glasses due to the red shift of their UV cut off. When the iron oxide content was increased to 1 mol% two bands could be observed in the visible region at 511, 662 nm and one near the infrared region at 949 (nm). Upon further increase of the iron oxide content to 3 mol % in glass F3, the band at 511 nm is obscured, while that at 949 nm becomes more developed. The band at 949 nm can be correlated with that due to Fe2+ ions appeared at 1050 nm in some sodium silicate glasses containing Fe2O3 [31, 34, 35]. The more development of the band at 949 nm in glass F3 indicates the increase of Fe2+ ions as the Fe2O3 content increases on the expense of Na2O. The band at 511 nm can be correlated with that recorded around 530 nm due to Fe3+ in lead oxyfluoro silicate glasses [6]. Its development at shorter wavelength in the present glasses can be attributed to the replacement of fluoride by oxygens legends. The band at 662 nm in the spectra of glasses F1 and F3 was assigned to Fe3+ in octahedral coordination [24].
From the optical measurements of sample with up to 3% Fe2O3 it can be noticed that iron ions exist as Fe3+ and Fe2+ states and the latter state increases as the Fe2O3 content increases on the expense of Na2O.
3.2 Infrared spectra
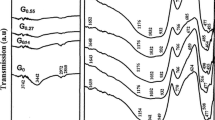
The transmission infrared spectra of the studied samples in the range 400–4000 cm −1 are illustrated in Fig. 2. The main structural vibrations are located in two regions: 400–600 and 600–1400 (cm −1).
The first region (Fig. 2b) includes complex band with peaks at 486 and 426 cm−1. The first band is due to bending Si–O–Si bonds in SiO4 tetrahedra [36, 37], while the peak at 426 cm−1 is attributed Fe–O bonds in FeO6 groups [38] overlapped with Pb–O vibrations in PbO4 units [39, 40]. The area under this complex band decreases and its peaks decrease in intensity as the Fe2O3 content increases which may be due to decrease in the number of Si–O–Si bond vibrations in SiO4 tetrahedra which may indicate a decrease in the SiO4 units. It can also noticed that the peaks at 486 cm−1 shifts to lower wavenumber at 465 cm−1 in sample F20 which may reflect loosening in the glass structure. The band at 426 cm−1 shows a shift to higher wavelengths as the Fe2O3 content increases. It reaches 440 cm−1 in sample F20. This behavior can be attributed to the formation of FeO4 at the expense of FeO6 [38].
In the second region (600–1400 cm −1) it can be noticed (Fig. 2b) that a symmetric stretching vibration of Si–O–Si bridging oxygen between tetrahedral SiO4 is demonstrated by the band at 618–605 cm−1 [41] in glasses with greater than 15 mol% Fe2O3 (F15, F16, F20). This band cannot be noticed in other samples due to the high intensity of the neighboring band in the range 754–762 cm−1. The band at 754 cm−1 (in glass F0) is assigned to Si–O–Si asymmetrical stretching vibration [42] overlapped with Si–O–Pb vibration [43]. The decrease of this band, particularly in samples F15, F16 and F20 enabled the appearance of the bands in the range 618−605 cm− 1. Asymmetric stretching vibration of Si-O-Si bonds in SiO4 units are demonstrated by the bands at 971 cm−1 [44, 45] and the bands in the region 1121−1054 cm−1 [46]. The bands in the range 855–927 cm−1 are assigned to asymmetrical stretching vibrations of Si–O terminals [45] overlapped with Si–O–Pb bonds [47]. The shift of the band at 855 cm−1 in glass F0 to higher frequency at 927 cm−1 in glass F20 can be attributed to the formation of non-bridging oxygens (NBO) with higher covalence.
From above discussion the SiO4 units dominate in sample F0 as demonstrated by the broad band in the region 600–1400 cm−1 (Fig. 2b). The decrease in the width of this broad band confirms the decrease of the SiO4 units upon substituting increasing amounts of Fe2O3 for Na2O.
Some absorption bands due to unavoidable adsorbed water molecules on the grain surfaces of the samples during their preparation for FTIR measurements are noticed. The bands at 1443 cm−1 for samples F0–F3 (Fig. 2b) are ascribed to bending modes of O–H [48]. This band is not recorded for samples with Fe2O3 higher than 7 mol%, which indicates better weathering resistant as the amount of Fe2O3 replacing Na2O increases. The 1640−1614 cm−1 (Fig. 2b) are attributed to bending modes of H–O–H [49] while those at 2362 cm−1 (Fig. 2a, b), 3462, 3479, and. 3782 cm−1 are attributed to stretching vibration bands of O–H in molecular H2O [50].
3.3 Experimental and theoretical densities
Figure 3a shows the experimental densities of the studied glasses. It can be noticed that the experimental density progressively increased up to sample F15 after which the increment rate of the density was increased with increasing iron oxide content up to F20. The theoretical density calculated using Huggins and Sun factors [51] shows (Fig. 3b) a progressive increase in density up to glass F20. This behavior differs from that of the experimental density, which may relate to changes in glass structure, particularly above 15 mol% Fe2O3. The calculated density depends mostly on the coordination geometry of the metal cations [51]. The weight factor may play the main role which caused the density to increase with higher rate. The observed deviation above 15 mol% Fe2O3 in the relation of density versus Fe2O3 content is in accordance with the observed variation of the FTIR behavior above 15 mol% Fe2O3. Table 2 lists the measured and the calculated theoretical density of the glasses studied.
3.4 XRD
The XRD of glass samples F7, F10, F15, F16 and F20 are presented in (Fig. 4). The first sample is representative for samples from F0 to F5. The XRD patterns of these samples indicate that they are mostly amorphous, however XRD patterns of samples F16 and F20 consist of amorphous halo and a few reflexes characteristic of crystalline phases in these samples. Small reflexes which correspond to the XRD pattern of hematite can be detected. In sample F20 and F16 the presence of lines at 2θ = 22.61°, 33.00°, 39.1°, 41.22° (41.84° for sample F16), 49.44°, 54.52°, 56.67° (59.90° for sample F16) are in good agreement with those of hematite α-Fe2O3 (JCPDS, PDF Nos., 85–0599 and 24–0072). The present findings are consistent with those obtained by other authors [52, 53]. From the experimental density (Fig. 3a) the increase in the increment rate of the density of samples with 15% Fe2O3 or more may indicate that denser glass structures are formed. The crystalline phases revealed by XRD may explain such increase in the higher rates in density. Accordingly, the formation of FeO4 units, as revealed by FTIR, and cross linking of the network of glass samples with more than 15 mol% Fe2O3 may enhance the formation of the hematite phase.
3.5 Electrical and dielectric properties
3.5.1 Dielectric behavior
Figure 5 shows the variation of dielectric constant with temperature as a function of Fe2O3 content, replacing Na2O at two frequencies 100 kHz and 1 MHz. The values of ε′ are almost constant for glass samples except for F16 and F20. The almost constant ε′ for glass samples from F0 to F15 indicates that the short range displacement of ions may be involved. The temperature dependence of ε′ for Fe2O3 > 15 mol% indicates that the movement of heavy dipoles was facilitated by weakening of bonds in the glass network with the increase of temperature [38]. The dielectric constant of samples F0, F0.25, F0.5, F1, F3, F5, F7, F10 and F15 shows weak variations. For example, they have average values of 12.16, 12.41, 12.75, 13.25, 14.09, 14.70, 14.78, 16.35, and 16.34, respectively at 1 MHz and temperature up to 300 °C, while for samples F16 and F20 the values of ε′ increase from −115 to 186 and from −34 to 66, respectively, at 1 MHz and temperature up to 300 °C.
Figure 6 shows the variation of dielectric constant with Fe2O3 content, replacing Na2O at the frequencies 50 Hz, 100 Hz, 500 Hz, 1 kHz, 10 kHz, 50 kHz, 100 kHz, 500 kHz, 800 kHz and 1 MHz, at two temperatures (25 and 300 °C). It can be noticed that the dielectric constant increases to a maximum with the increasing of the iron oxide content. For example, for sample F0 the ε′ increases from 13.90 to reach a maximum value of 132.60 for sample F16 then it decreases to 43.87 at 100 kHz and 25 °C. This decrease in the dielectric constant after reaching maximum values with increasing Fe2O3 > 15 mol% can be attributed to the decrease of Fe3+ ions contributing in the conduction mechanism as a result of increasing the proportion of Fe3+ states in network forming positions [54]. The present attribution is consistent with the above obtained IR results where more Fe3+ ions are likely to be introduced as NWF in glasses with 20 mol% Fe2O3.
From Fig. 6 it can be noticed that the values of ε′ at 100 kHz or lower frequencies are generally higher than those at higher frequencies up to 1 MHz (Fig. 6) particularly for glasses F16 and F20. It is worth noticing that the dielectric constant decreases as the measurement is carried out at higher frequencies, which can be attributed to the slow response of the heavy dipoles behind the external field [38, 55, 56].
3.5.2 Electrical conductivity
The introduction of transition metal ions (TMI) with more than one valence state in glass exhibited semiconducting properties [57–59], where conduction can occur by electron transfer from ions in the lower valence state to those in a higher valence state [58, 60]. Figure 7 shows the variation of the logarithm of ac conductivity of the glasses studied as a function of inverse temperature (T) at 100 kHz and 1 MHz. It can be seen that the conductivity increases by raising the temperature. The plots of logσac versus inverse temperature show two parts at low and high temperature regions. The values of the activation energy of those in the high temperature region have been determined from their slopes and listed Table 2. From this table it can be seen that the activation energy is significantly reduced from the range 2.37–1.74 eV to the range 0.36–0.16 eV for samples F0 and F16, respectively, then it is increases to the range 1.91–1.56 eV for sample F20. The values of activation energies for glasses F0, F0.25, F0.5, F1 and F3 reveal that their conduction is mainly ionic in nature, while glasses F5, F7, F10, F15 and F16 have lower values indicating the dominant of electronic conduction. The lowest activation energy of glass F16 which reached 0.36 eV (at 100 kHz) or 0.16 eV (at 1 MHz), can be attributed to the dominant and the increase of the electron charge carriers. For glass F0 the high activation energy (2.37 or 1.74 eV at 100 kHz and 1 MHz, respectively), can be explained on the bases of the above obtained FTIR results. The dominate of the SiO4 units with bridging oxygens and the absence of iron ions in the composition of this glass (F0) results in the higher energy needed for migration of the charge carriers. The conduction seems to proceed via electronic at the low temperature regions and via ionic conduction in the high temperature region. These results are consistent with published data [61]. The increase of the electronic conduction upon increasing the iron oxide content above 1 mol% seems to be related to the more increase of Fe2+ ions. When glass containing Fe2O3 is melted at high temperature, part of ferric ions (Fe3+) are converted into the divalent state (Fe2+): Fe3+ → Fe2+ + e. Usually, iron occurs in silicate glasses in equilibrium between Fe3+ and Fe2+ ions, where their ratio Fe2+/Fe3+ is a function of glass composition, pressure, temperature and oxygen fugacity [62]. When melting of such glasses is carried out in the presence of a reducing material such as Pb [5], the prepared glasses are expected to contain relatively high Fe2+ content, consequently the electron hopping between Fe2+ and Fe3+ Increases. The increased activation energy in the high temperature region for glass F20 (Table 2) to values of 1.91 or 1.56 eV can be attributed to the re-participation of the ionic conduction which needs relatively high activation energy.
Figure 8 shows that the conductivity of the glass samples increases with increasing of Fe2O3 content at 25 and 300 °C. The increase in ac conductivity with increasing Fe2O3 content that replacing Na2O can be attributed to the increase of charge carriers. It can be noticed that the increase in conductivity is accompanied by a decrease in activation energy (Table 2) with increasing Fe2O3 content. This behavior is consistent with several published data for glasses containing transition metal oxide [63–65]. At 100 kHz and at 25 °C, the conductivity is increased from 6.24 × 10−7 to 9.69 × 10−4 S/cm for samples F0 and F20, respectively, while at 300 °C it increased from 2.02 × 10−5 to 2.15 × 10−2 S/cm for samples F0 and F20, respectively. The conduction in the present glasses—containing Fe2O3− seems to be a combination of ionic and electronic controlled by electron hopping between Fe2+ and Fe3+ sites. It can also be noticed (Fig. 8a, b) that the conductivity increases with increasing the frequency. The increase of conductivity with increasing frequency confirms the semiconducting nature of the studied glasses.
4 Conclusions
Glass of the composition (mol%) (25–x) Na2O−xFe2O3–25PbO–50SiO2, where x = 0, 0.25, 0.5, 1, 3, 5, 7, 10, 15, 16 and 20 (mol%) were prepared by the melt quenching method.
Replacing Fe2O3 for Na2O affect the optical properties as a result of the contribution of Pb2+, Fe2+ and Fe3+ ions. Absorption bands due Fe3+ and Fe2+ oxidation states are recorded in the visible and near IR regions. The optical measurements revealed that iron ions exist as Fe2+ state increases, as the Fe2O3 content increases at the expense of Na2O.
The FTIR indicates that replacement of Na2O by ≥15 mol% Fe2O3 produces structural changes, where the SiO4 units decrease and FeO4 units increase. It also indicates that samples with better weathering resistant are obtained as the amount of Fe2O3 replacing Na2O increases.
XRD indicated change in the amorphous state after replacement Na2O by ≥15 mol% Fe2O3, where the sample with Fe2O3 content of 20 mole% exhibits crystals of hematite phase to be formed in a glassy matrix.
The electrical conductivity vs. reciprocal temperature revealed two parts at low and high temperature region. So, electrical conduction in the studied glasses seems to proceed by ionic and electronic controlled by electron hopping between Fe2+ and Fe3+ sites. The activation energy of electrical conduction at the high temperature region decreased from 1.74 eV for glass sample of un-replaced Na2O to 0.16 eV (at 1 MHz) as the amount Fe2O3 replacing Na2O was increased to 16 mol% Fe2O3. The electrical conduction is mainly electronic in nature as the Na2O replaced by Fe2O3 increases to 16 mol% and above this concentration both mechanisms the ionic and electronic proceed.
The dielectric constants of samples with replaced Na2O < 16 mol% Fe2O3 show weak variation which reveal high thermal stability in the studied range of temperature. They have average values in the range of 12–16 measured at 1 MHz and temperature up to 300 °C. Samples with 16 and 20 mol% Fe2O3 show high ε′ values of 115 to 186 and from 34 to 66, respectively, at 1 MHz and temperature up to 300 °C. These results represent promising candidates for the studied glasses to be used as energy storage materials in electronic devices.
References
G.P. Kothiyal. S.V. Phadnis, V.K. Shrikhande, T. Mirza, M.K. Totlani, V.C. Sahni, Anti-Corros Methods Mater. 47, 280 (2000).
S.P. Singh, R.P.S. Chakradhar, J.L. Rao, B. Karmakar, J. Alloys Compd. 493, 256 (2010)
T. Komatsu, Mohiri H., Phys. Chem. Glasses 40, 257 (1999).
A. M. Zahra, Zahra C.Y., Piriou B., J. Non-cryst. Solids 155, 45 (1993).
N.K. Sanjay, A. Agarwal, Ind. J. Pure Appl. Phys 48, 205 (2010)
T.G.V.M. Rao, A. R. Kumar, B. H. Rao, N. Veeraiah, M. R. Reddy, J. Molec. Struct. 1021, 7 (2012).
P.W. Wang, L. Zhang, J. Non-Cryst. Solids 194, 129 (1996)
J. Rybicki, A. Rybicka, A. Witkowska, G. Bergmanski, A. Di Cicco, M. Minicucci, G. Mancini, J. Phys. 13, 9781 (2001)
P.R. Rao, L. Pavić, A. Moguš-Milanković, V. Ravi Kumar, I.V. Kityk, N. Veeraiah, J. Non-Cryst. Solids 358, 3255 (2012)
N.C.R. Babu, M.A. Valente, N.N. Rao, M.P.F. Graça, G.N. Raju, M. Piasecki, N. I.V. Kityk, Veeraiah. J. Non-Cryst. Solids 358, 3175 (2012)
B. Mehdikhani, G. H. Borhani, Process. Appl. Ceram. 7, 117 (2013).
B.O. Mysen, D. Virgo, E.R. Neumann, F.A. Seifert, Am. Mineral 70, 317 (1985)
T. Uchino, K. Nakaguchi, Y. Nagashima, T. Kondo, J. Non-Cryst. Solids 261, 72 (2000)
B.O. Mysen J. Non-Cryst. Solids 95–96, 247 (1987)
B. Hannoyer, M. Lenglet, D. Durr, R. Cortes, J. Non-Cryst. Solids 151, 209 (1992)
C. Weigel, L. Cormier, G. Calas, D.T. L.e Galoisy, Bowron. J. Non-Cryst. Solids 354, 5378 (2008)
G. Ottonello, R. Moretti, L. Marini, M.V. Zuccolin, Chem. Geol. 174, 157 (2001)
J.A. Tangeman, R. Lange, L. Forman, Geochim. Cosmochim. Acta 65, 1809 (2001)
R. Klement, J. Kraxner, M. Liška, Ceramics–Silikáty 53, 180 (2009).
F. Abdel-Wahab, A.G. Mostafa, A.E. Belal, E.M. El-Agwany, Mater. Chem. Phys. 93, 243 (2005)
D.P. Singh, G.P. Singh, J. Alloys Compd. 546, 224 (2013)
J. Coelho, C. Freire, S. N. Hussain. Spectrochimica Acta Part A 86, 392 (2012)
R.P.S. Chakradhar, G. Sivaramaiah, J.L. Rao, N.O. Gopal. Spectrochimica Acta Part A 62, 51 (2005)
M. Ookawa, T. Sakurai, S Mogi, T. Yokokawa, Mater Trans, JIM, 38, 220 (1997).
R.J. Barczyński, N.A. Szreder, J. Karczewski, M. Gazda, Solid State Ionics 262, 801 (2014)
F. Pinakidou, M. Katsikini, P. Kavouras, F. Komninou, Th. Karakostas, E. C. Paloura, J. Non- Cryst. Solids, 354, 105 (2008).
H. Hagiwara, R. Oyamada, Yogyo-Kyokai-Shi 84, 264 (1976).
S. Ibrahim, Morsi M. Morsi, Mater. Chem. Phys. 138, 628 (2013)
T. Sankarappa, M. Prashant Kumar, G.B. Devidas, N. Nagaraja, R.R. Reddy, J. Mol. Struct. 889, 308 (2008)
B. Kumar, T. Vijaya, M. Sankarappa, S. Kumar, P.J.P. Kumar, R. Sadashivaiah, R. Reddy, Phys. B 404, 3487 (2006).
C.R. Banford, Glass Science and Technology, Colour Generation and Control in Glass. (Elsevier, Amsterdam, 1977)
W.A. Weyl, Society of Glass Technology, Coloured Glasses London, (1951)
B. Mirhadi, J. B. Mehdikhani, Korean Ceram. Soc. 48, 117 (2011)
S.B. Donald, A.M. Swink, H.D. Schreiber, J. Non-Cryst. Solids, 352, 539 (2006).
K.R. Kukadapu G.L Li H. Smith, J. Non-Cryst.. Solids 317, 301(2003).
S.A. MacDonald, C.R. Schardt, D.J. Masiello, J.H. Simmons, J. Non-Cryst. Solids 275, 72 (2000)
A.M.B. Silva, C.M. Queiroz, S. Agathopoulos, R.N. Correia, M.H.V. Fernandes, J.M. Oliveira, J. Mol. Struct. 986, 16 (2011)
R. Hisam, A.K. Yahya, H. M. Kamari, Z. A. Talib. R. H. Y. Subban Mater. Express, 6, 149 (2016).
M. Bosca, L. Pop, B. Ghe, P. Pascuta, E. Culea, J. Alloys Compd. 479, 579 (2009)
O.C. Mocioiu, M. Popa, E.I. Neacsu, M. Zaharescu, J Non-Cryst. Solids. 361, 130 (2013).
G. Kaur, M. Kumar, A. Arora, O.P. Pandey, K. Sinh, J. Non-Cryst. Solids 357, 858 (2011)
R. D. Husung Robert H. Doremus, J. Mater. Res. 5, 2209 (1990).
B. Karmakar, R.N. Dwivedi, J. Non-Cryst. Solids 342, 132 (2004)
M.T. Wang, J.S. Cheng, M. Li, F. He, Physica B 406, 187 (2011)
L. Stoch, M. S´roda, J. Molec. Struct. 511–512511–512, 77 (1999).
E.C. Ziemath, M.A. Aegerter, J. Mater. Res. 9, 216 (1994)
T. A. Sidorov, Zhurnal Prikladnoi Spektroskop ii 6, 98(1967).
H. Moor, P.W. McMillan, J. Soc. Glass Technol. 40, 97 (1956).
C.I. Merzbacher, W.B. White, J. Non-Cryst. Solids 130, 18–34 (1991)
G. Navara, J. Non Cryst Solids 353, 555 (2007)
M.L. Huggins, K. H. Sun, J. Am.Ceram.Soc.26,4(1943), in Handbook of Glass Manufacture (Compiled and Edited by F.V. Tooley, Ogdin Pub. Co. ,N.Y.(1953) P.25
M. S. Islam, J. Kurawakia, Y. Kusumoto, M. A. Al-Mamun, M. Z. Bin Mukhlish, J. Sci. Res. 4, 99(2012).
S.K. Sahoo, K. Agarwal, A.K. Singh, B.G. Polke, K.C. Raha, Int. J. Eng. Sci. Technol. 2, 118 (2010)
S. Yusub, G.S. Baskaran, S.B.M. Krishna, C.H. Rajyasree, A.R. Babu, D.K. Rao, Ind. J. Pure Appl. Phys. 49, 315 (2011)
N.D. Sankyr, E. Aydyn, M. Sankyr, Int. J. Electrochem. Sci. 9, 3864 (2014)
R.M.M. Morsi, A.M. Abdelghany, M.M. Morsi, J Mater Sci. 26, 5120 (2015)
M.A. Sidkey, A.A. El-Moneim, L.A. El-Latif, Mater. Chem. Phys. 61, 103 (1999)
M.M. El-Desoky, J. Non-Cryst. Solids 351, 3139 (2005)
V. Rajendran, N. Palanivelu, B.K. Chaudhuri, K. Goswami, J. Non-Cryst. Solids 320, 195 (2003)
S. Annamalai, R.P. Bhatta, I.L. Pegg, B. Dutta, J. Non- Cryst. Solids 358, 1380 (2012)
A.G. Mostafaa, A.H. El-Dosokey, K.H. Idress, H.M. Goumaa, Int. J. Res Eng. Sci. (IJRES) 2, 58 (2014)
E.S. Dunaeva, I.A. Uspenskaya, K.V. Pokholok, V.V. Minin, N.N. Efimov, E..A. Ugolkova, E. Brunet, J. Non-Cryst.Solids 358, 3089 (2012).
A. Al- Hajry, A.A. Soliman, M.M. El- Desoky, Thermochim Acta 427, 181 (2005)
M.M. El-Desoky, J. Mater. Sci. 14, 215 (2003)
M.M. El-Desoky, I. Kashif, Phys. Stat. Sol. (a) 194, 89 (2002)
Acknowledgements
The authors acknowledge the financial support of this work from the National Research Centre, 33 E l Bohoth St. (former El Tahrir St.), Dokki, Giza P.O. 12622, Egypt, under Contract No. P 100910/ 2015–2016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morsi, R.M.M., Ibrahim, S. & Morsi, M.M. Characterization of sodium lead silicate glasses containing low and high levels of Fe2O3 and effect of its replacement for Na2O. J Mater Sci: Mater Electron 28, 9566–9574 (2017). https://doi.org/10.1007/s10854-017-6704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6704-1