Abstract
In this investigation, a facile precipitation route has been developed for the preparation of cadmium tungstate (CdWO4) nanoparticles by using cadmium nitrate hexahydrate and sodium tungstate dehydrate as cadmium and vanadate precursor, respectively. The effect of different capping agents such as glycine, valine, and alanine on the morphology and particle size of the products has been investigated. Facile preparation and separation are important features of this route. This work has provided a general, simple, and effective method to control the composition and morphology of cadmium tungstate in aqueous solution, which will be important for inorganic synthesis methodology. The morphology and particle size of products were investigated by scanning electron microscopy images, X-ray diffraction patterns, ultraviolet–visible and electron dispersive X-ray spectroscopy spectroscopy. The photocatalytic characteristics of as-obtained nanocrystalline cadmium tungstate were also examined by degradation of methyl orange dye as water contaminant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanostructures have gained much attention among materials, because the nanocrystal properties not only depend on their composition but also depend on their size, shape, and size distribution. Recently, there are extensive researches on the environmental problems, especially photocatalytic applications due to their high applicability in the society. The decolorization of hazardous organic dyes such as rhodamine B, methylen blue, and methyl orange are very important, because their discharge from industrial applications causes severe adverse effects to the environment and greatly increases the water pollution and hence effectively disturbs the ecosystem [1–8]. In recent years, tungstates and molybdates are materials that have attracted the interest of many researchers due to their broad potential to the industrial application including optic fiber, humidity sensor, catalysts, scintillation detector, solid-state lasers, photoluminescent devices, microwave applications, and so on [9–14]. Molybdates and tungstates are significant luminescent materials with scheelite-type tetragonal structure, membership I41/a space group with two formula units per primitive cell. Every of X atoms X = Mo and W) is enclosed by four equivalent O atoms composing the [XO4]2 tetrahedral configuration and every divalent metal shares corners with eight adjacent O atoms of [XO4]2 tetrahedrons [15–19]. Various methods have been employed to synthesize metal molybdates, such as solid-state reaction, electrochemical methods, the chochralski technique, hydrothermal method, solvothermal synthesis, and co-precipitation [20–24]. Precipitation processing has been proven to be a useful technique for generating novel materials with unusual properties. In this work, we report the synthesis and characterization of cadmium tungstate (CdWO4) nanoparticles through the low-cost, fast, cheap, and easily controllable precipitation method. Moreover, the impact of different capping agents such as glycine, valine, and alanine on the morphology and particle size of final products was examined. The photocatalytic activity of CdWO4 nanoparticles was evaluated by degradating of methyl orange.
2 Experimental
2.1 Characterization
All the chemicals used in this method were of analytical grade and used as-received without any further purification. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. The UV–vis diffuse reflectance spectrum of the as prepared nanocrystalline cadmium tungstate was obtained on a UV–vis spectrophotometer (Shimadzu, UV-2550, Japan). The EDS analysis was carried out by a Philips XL30 microscope. Powder X-ray diffraction (XRD) pattern of the as-obtained nanocrystalline cadmium tungstate was recorded by applying a diffractometer of Philips Company with X’Pert Promonochromatized Cu Ka radiation (k = 1.54 A °). The magnetic measurement of samples were carried out in a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran) at room temperature in an applied magnetic field sweeping between ± 10,000 Oe.
2.2 Synthesis of CdWO4 nanoparticles
CdWO4 nanoparticles were synthesized by a simple co-precipitation method. In a typical procedure, an aqueous solution of Na2WO4·2H2O in the presence of different capping agents, such as glycine, valine, and alanine was mixed with Cd(NO3)2·6H2O aqueous solution and solution heated up to 70 °C for 15 min. The white product was filtered, washed with distilled water and methanol for several times and dried in vacuum at 60 °C. Finally, the white precipitate was filtered and washed three times with distilled water. The final product was dried at 90 °C and then calcined at 500 °C for 120 min in a conventional furnace in air atmosphere. Reaction conditions are listed in Table 1.
2.3 Photocatalytic experimental
The photocatalytic activities of CdWO4 nanoparticles were determined by the degradation of aqueous methyl orange (MO) under UV light. About 0.1 gr of the sample was first inserted into a reactor that included 50 ppm of aqueous MO. The suspension was transferred into a self-designed glass reactor, and stirred in darkness to attain the adsorption equilibrium. In the research of photo degradation by UV light, a 400 W mercury lamp with a water cooling cylindrical jacket was utilized. The photocatalytic activity of CdWO4 nanoparticles was tested by using MO solution. The degradation reaction was carried out in a quartz photocatalytic reactor. The photocatalytic degradation was carried out with 50 ppm of MO solution containing 0.1 g of CdWO4 nanoparticles. This mixture was aerated for 30 min to reach adsorption equilibrium. Then, the mixture was placed inside the photoreactor in which the vessel was 20 cm away from the UV. The quartz vessel and light sources were placed inside a black box equipped with a fan to prevent UV leakage. Aliquots of the mixture were taken at periodic intervals during the irradiation, and after centrifugation they were analyzed with the UV–Vis spectrometer. The MO degradation percentage was calculated as:
where At and A0 are the obtained absorbance value of the MO solution at t and 0 min by a UV–vis spectrometer, respectively.
3 Results and discussion
In the third millennium, current studies show that different type of capping agent such as ionic, polymeric; etc play a fundamental role in synthesis procedures [10, 25, 26]. Capping agents and surfactants are used in inorganic chemistry to prevent particle agglomeration by reducing condensation reactions in liquid phase synthesis. In this research glycine, valine, and alanine used as surfactant to investigate their effect on particle size of products at constant calcinations temperature 500 °C. Figure 1 a–c shows the SEM images of CdWO4 nanoparticles in the presence glycine, valine, and alanine as the capping agent accordance with sample 1–3, respectively. According to the Fig. 1 a and b, in the presence glycine and valine product mainly consists of spherical shape nanoparticles with average particle size 40–80 nm. Furthermore, in the presence of glycine and valine as capping agent products have smaller size than alanine as the capping agent.
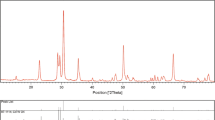
The powder X-ray difraction technique was used to evaluation of crystal structure and phase purity of CdWO4 nanoparticles and the data has been shown in Fig. 2. The XRD peaks demonstrated a well match with the pure monoclinic phase of CdWO4 with space group of P2/c and JCPDS no. 87-1114. Also no additional peaks were observed because of purity of samples.
By the Debye–Scherrer approximation (Eq. 2) the crystallite size of CdWO4 nanoparticles were calculated from XRD data as 10 nm.
where β is the breadth of the observed diffraction line at its half intensity maximum, K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. The purity of nanocrystalline product was also confirmed by EDS analysis (Fig. 3). According to Fig. 3, the sample no. 1 is composed of Cd, W and O elements. Furthermore, no impurity peaks are seen, which indicates a high level of purity in the sample. The magnetic properties of nanoparticles that calcined at 500 °C have been studied by VSM method (Fig. 4; sample no. 1). The VSM data demonstrated that the CdWO4 nanoparticles have paramagnetic property and the magnetize saturation of products is about 0.004 emu/g and a coercivity of 95 Oe at room temperature. Figure 5 shows the (αhʋ)2 vs hy curve of CdWO4 nanoparticles which were calculated from their UV–vis absorbance using the equation proposed by Wood and Tauc exhibited the equation below.
where a is the absorbance, h the Planck constant, y the photon frequency, Eg the energy gap, and n the pure numbers associated with the different types of electronic transitions. For n = 1/2, 2, 3/2 and 3, the transitions are directly allowed, indirectly allowed, directly forbidden, and indirectly forbidden, respectively. Each energy gap was determined by extrapolation of each linear portion of the curves to a = 0. In the present research, the CdWO4 presents directly allowed electronic transition (n = 1/2) and the energy gaps of CdWO4 nanoparticles is 3.05 eV. Photodegradation of MO as water contaminant under UV light illumination was employed to evaluate the properties of the as-synthesized CdWO4 nanoparticles. Figure 6 exhibits the obtained result. No methyl orange was practically broken down after 70 min without employing UV light illumination or as-prepared nanoparticles CdWO4. This observation illustrated that the contribution of self-degradation was insignificant. The proposed mechanism of the photocatalytic degradation of the methyl orange can be assumed as:
Utilizing photocatalytic calculations by Eq. (1), the methyl orange degradation was about 73% after 70 min illumination of UV light in the presence of samples 1. This obtained result demonstrates that as-prepared CdWO4 nanoparticles have high potential to be applied as favorable and appropriate material for photocatalytic applications under illumination of UV light. The heterogeneous photocatalytic processes have diffusion, adsorption and reaction steps. It has been shown that the desirable distribution of the pore has effective and important impact on the diffusion of the reactants and products, and therefore effects on the photocatalytic activity. It seems that the enhanced photocatalytic activity of the as-obtained nanoparticles CdWO4 can be owing to desirable and appropriate distribution of the pore, high hydroxyl amount and high separation rate of charge carriers (Scheme 1). Furthermore, this route is facile to operate and very suitable for industrial production of CdWO4 nanoparticles.
4 Conclusions
CdWO4 powders were synthesized by a precipitation method. Glycine, valine, and alanine were used as surfactant. CdWO4 nanocrystals were characterized by XRD, SEM, EDX and UV–Vis spectroscopy. Vibrating sample magnetometer analyzes indicates a ferromagnetic behavior for the synthesized nanoparticles. The photocatalytic behavior of nanoparticles was evaluated using the degradation of methyl orange aqueous solution under UV light irradiation. The results show that CdWO4 nanoparticles are good candidate with excellent performance in photocatalytic applications for degradation of dyes under UV irradiation in a short time.
References
M. Zahraei, A. Monshi, D. Shahbazi-Gahrouei, M. Amirnasr, B. Behdadfar, M. Rostami, J. Nanostruct. 5, 137 (2015)
M. Hamadaniana, H. Sayahi, A. R. Zolfagharici. J. Nanostruct. 1, 237 (2012)
S.M. Hosseinpour-Mashkani, A. Sobhani-Nasab, M. Maddahf, J. Nanostruct., 6, 67 (2016)
M. Shahrekizad, A. Gholamalizadeh-Ahangar, N. Mir, J. Nanostruct. 5, 117 (2015)
F. Beshkar, M. Salavati-Niasari, J. Nanostruct. 5, 17 (2015)
M. Panahi-Kalamuei, M. Mousavi-Kamazani, M. Salavati-Niasari, J. Nanostruct. 4, 459 (2014)
M.P. Mazhari, A. Abbasi, A. Derakhshan, M. Ahmadi J. Nanostruct. 1, 99 (2016).
T. Poursaberi, H. Ghanbarnejad, V. Akbar, J. Nanostruct 2, 417 (2013)
M. Malekshahi Byranvand J. Nanostruct. 1, 1 (2016)
R. Talebi, J. Mater. Sci. 6, 5665 (2016)
R. Talebi, S. Khademolhoseini, S. Rahnamaeiyan, J. Mater. Sci. 27, 1427 (2016)
S. S. Hosseinpour-Mashkani, S. S. Hosseinpour-Mashkani, A. Sobhani-Nasab J. Mater. Sci. 27, 4351 (2016).
A. Sobhani-Nasab, M. Sadeghi J. Mater. Sci. 27, 7933 (2016).
A. Sobhani-Nasab, M. Behpour J Mater Sci 27, 1191 (2016)
A. Phuruangrat, T. Thongtem, S. Thongtem, J. Ceram. Soc. Jpn. 116, 605 (2008)
L.S. Cavalcante, J.C. Sczancoski, R.L. Tranquilin, M.R. Joya, P.S. Pizani, J.A. Varela, E. Longo, J. Phys. Chem. Solids 69, 2674 (2008)
L.S. Cavalcante, J.C. Sczancoski, J.W.M. Espinosa, J.A. Varela, P.S. Pizani, E. Longo, J. Alloys. Compd. 474, 195 (2009)
L.S. Cavalcante, J.C. Sczancoski, L.F. Lima Jr., J.W.M. Espinosa, P.S. Pizani, J.A. Varela, E. Longo, Cryst. Growth. Des. 9, 1002 (2009)
J.C. Sczancoski, L.S. Cavalcante, M.R. Joya, J.A. Varela, P.S. Pizani, E. Longo, Chem. Eng. J. 140, 632 (2008)
Z.H. Li, J.M. Du, J.L. Zhang, T.C. Mu, Y.N. Gao, B.X. Han, J. Chen, J.W. Chen, Mater. Lett. 59, 64 (2005)
Y.M. Zhang, F.D. Yang, J. Yang, Y. Tang, P. Yuan, Solid State Commun. 133, 759 (2005)
S.M. Hosseinpour-Mashkani, M. Maddahfar, A. Sobhani-Nasab, J. Electron. Mater. 45, 3612 (2016)
P. Yang, G.Q. Yao, J.H. Lin, Inorg. Chem. Commun. 7, 389 (2004)
S.M. Hosseinpour-Mashkani, A. Sobhani-Nasab, J. Mater. Sci. Mater Electron. 27, 7548 (2016)
M. Maddahfar, M. Ramezani, M. Sadeghi, A. Sobhani-Nasab, J. Mater. Sci. Mater. Electron. 26, 7745 (2015)
M. Behpour, M. Mehrzad, S.M. Hosseinpour-Mashkani, J. Nanostruct. 5, 183 (2015)
Acknowledgements
Authors are grateful to council of University of Central Tehran for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vosoughifar, M. Influence of capping agents additives on morphology of cadmium tungstate nanoparticles and study of their photocatalytic properties. J Mater Sci: Mater Electron 28, 6800–6805 (2017). https://doi.org/10.1007/s10854-017-6377-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6377-9