Abstract
The Li3Mg2NbO6 ceramics doped with ZnO-B2O3-SiO2 (ZBS) additives were synthesized via the conventional solid-state reaction process. The influence of ZBS additives on phase composition, sintering behavior, microstructure and microwave dielectric properties of Li3Mg2NbO6 ceramics were investigated in detail. The XRD patterns showed that the sintered specimen presented a single phase and no secondary phase appeared. We found that proper amount of ZBS additives could significantly reduce the sintering temperature from 1250 to 925 °C and promote the densification of Li3Mg2NbO6 ceramics. The εr and Q × f value were strongly affected by bulk density and grain size, respectively. As ZBS content increased, the τf value shifted toward negative direction. In summary, excellent microwave dielectric properties of εr ~ 14.84, Q × f ~ 73,987 GHz, τf ~ −16.05 ppm/°C could be obtained in 0.5 wt.% ZBS modified sample when sintered at 925 °C for 4 h. Furthermore, the material was compatible with Ag electrode, demonstrating that it would be a promising candidate material for LTCC application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microwave dielectric ceramics have played a significant role in modern microwave communication and telecommunication system in recent years [1–4]. These materials are often used to fabricate passive components in microwave circuits, such as resonators, filters, oscillators and antennas. For practical application, three parameters including a proper dielectric constant, a high quality factor and a near zero temperature coefficient of resonant frequency should be taken into account for microwave ceramics. To meet the requirement of LTCC, it is necessary to reduce the sintering temperature to below 961 °C.
Li3Mg2NbO6 ceramics were firstly reported by Yuan et al. [5] and exhibited good microwave dielectric properties of εr ~ 16.8, Q × f ~ 79,643 GHz, τf ~ −27.2 ppm/°C. Then, Wu et al. [6] reported the characterization of Li3Mg2NbO6 ceramics based on chemical bond theory and the maximum Q × f value of 100,965 GHz was achieved in a saturated sample when sintered at 1225 °C for 4 h. Later, Zhang et al. [7] investigated the microstructure and microwave dielectric properties of Li3(Mg0.95A0.05)2NbO6 (A = Ca2+, Ni2+, Zn2+, Mn2+) ceramics and preferable microwave dielectric properties of ε r ~ 15.62, Q × ƒ ~ 96,160 GHz, τ ƒ ~ −18.49 ppm/°C were realized in Li3(Mg0.95Ca0.05)2NbO6 ceramics when sintered at 1140 °C for 4 h. The higher Q × ƒ value of Li3Mg2NbO6-based ceramics was favorable to microwave components in the selectivity of operating frequency, but the high sintering temperature limited its further applications in microwave device. Therefore, Zuo et al. [8, 9] studied and concluded that 0.5 wt.% 0.17Li2O-0.83V2O5 could effectively reduce the sintering temperature of Li3(Mg0.92Zn0.08)2NbO6 ceramics to 925 °C and possessed good microwave dielectric properties of ε r ~ 14, Q × ƒ ~ 83,395 GHz, τ ƒ ~ −37.2 ppm/°C. They also investigated the impact of Ba3(VO4)2 additives on microwave dielectric properties of Li3(Mg0.92Zn0.08)2NbO6 ceramics and found that 0.7Li3(Mg0.92Zn0.08)2NbO6–0.3Ba3(VO4)2 ceramics sintered at 950 °C had a near zero temperature coefficient of resonant frequency(τ ƒ ~ +1.5 ppm/°C).
Recently, Liao et al. [10] used B2O3 to reduce the sintering temperature of Li3Mg2NbO6 ceramics to 925 °C, but its Q × f value was seriously impaired. Until now, there were few reports about lowering temperature of Li3Mg2NbO6 ceramics. Generally, low-melting glasses were often used as promising sintering aid to decrease the sintering temperature of ceramics, such as MgO-B2O3-SiO2, Li2O-B2O3-SiO2, ZnO-B2O3-SiO2 [11]. And ZBS additives have been used and successfully decreased the sintering temperature in many ceramics [12, 13]. However, the effect of ZBS additives on sintering behavior and microwave dielectric properties of Li3Mg2NbO6 ceramics have not been studied yet.
Therefore, in this paper, ZBS additives was added into Li3Mg2NbO6 ceramics to lower the sintering temperature. Moreover, the phase structure, microstructure and microwave dielectric properties of ZBS added Li3Mg2NbO6 ceramics were investigated systemically.
2 Experimental procedure
Li3Mg2NbO6 + xwt.% ZBS (x = 0.1–3.0) ceramics were synthesized through a conventional solid state reaction method. Firstly, high purity oxide powders of Li2CO3 (98.0%), MgO (99.0%), Nb2O5 (99.9%) were weighed in stoichiometric amounts and ball-milled with distilled water for 6 h by using ZrO2 balls. Next, the slurry was dried and calcined at 1050 °C for 4 h to synthesize pure Li3Mg2NbO6 powders. Later, various amounts of ZBS (60 wt.% ZnO, 30 wt.% B2O3, 10 wt.% SiO2) additives were added into calcined powders. Then, they were re-milled with distilled water for 6 h, after drying and sieving, the ground powders mixed with 8 wt.% paraffin as a binder, granulated and pressed into pellets with dimension of 10 mm in diameter and 5 mm in thickness. Finally, the obtained pellets were sintered at 850 – 950 °C for 4 h with the heating rate of 5 °C/min.
The crystalline phase of sintered samples were identified by X-ray diffraction (XRD, Rigaku D/max 2550 PC, Tokyo, Japan). The microstructure was analyzed by a scanning electron microscope (SEM, ZEISS MERLIN Compact, Germany). While microwave dielectric properties [14] of sintered specimen were measured by a network analyzer (N5234A, Agilent Co., America) in the frequency range of 7–13 GHz. The τf values were measured in the temperature range from 25 to 85 °C. It was calculated by the following formula:
where f 1 and f 2 was the corresponding frequency at the temperature of T 1 and T 2 respectively.
3 Results and discussion
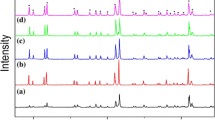
Figure 1 shows the XRD patterns of Li3Mg2NbO6 + xwt.% ZBS (x = 0.1–3.0) ceramics sintered at 925 °C for 4 h. We can observe that all the main peaks could be indexed based on JCPDS file86-0346 for Li3Mg2NbO6 phase with orthorhombic structure. And no additional phase arise in the XRD patterns, which indicate that ZBS additives are nonreactive with Li3Mg2NbO6 ceramics and only exist as liquid phase in the sample.
Figure 2 illustrates the SEM images of Li3Mg2NbO6 + xwt.% ZBS (x = 0.1–3.0) ceramics sintered at various temperatures. The micro-morphology variations of Li3Mg2NbO6 ceramics with different amounts of ZBS additives sintered at 925 °C are shown in Fig. 2a–e. It can be seen that the specimen doped with 0.1wt.% ZBS is not dense and some pores can be observed clearly (Fig. 2a), with the increase of ZBS content, the grains grow rapidly and became bigger, the pores disappear. At the level of 0.5wt.% ZBS additives, well dense and uniform distribution with the grain size of 10–15 um can be obtained in Fig. 2b. However, the composition with 1.0wt.% ZBS shows apparently abnormal grain growth, causing an uneven surface and some relatively smaller grains, as shown in Fig. 2c. Continue increasing ZBS additives would significantly decrease the grain size and lead to a porous microstructure as shown in Fig. 2d, e. Thus, proper amounts of ZBS additives play an critical role in promoting the grain growth and improving the density of Li3Mg2NbO6 ceramics, which may be correlated with the liquid phase mechanism during sintering process [15]. Whereas, excessive ZBS additions are harmful to the densification of Li3Mg2NbO6 ceramics and would decrease the grain size.
Moreover, to study the variation of grain growth with sintering temperature, SEM micrographs of 0.5wt.% and 1.0wt.% ZBS modified Li3Mg2NbO6 ceramics sintered at 900 °C and 950 °C are illustrated (Fig. 2f–i). We can notice that the compositions with 0.5wt.% ZBS sintered at 900–950 °C exhibit a dense microstructure, the grains grow quickly and exhibit a more uniform distribution when temperature rise from 900 to 925 °C (Fig. 2b, f), further raising temperature would result in a inhomogeneous grain size (Fig. 2g). Similar phenomenon is found in the 1.0 wt.% ZBS added Li3Mg2NbO6 ceramics, the difference is that all the specimens with 1.0 wt.% ZBS have comparatively smaller grains(3–8 um), and aberrant grain morphology appeared in the compound when sintered at 925 °C. For 950 °C (Fig. 2i), we can see that some grains have melted, leading to the indistinct grain boundaries which would hardly deteriorate the microwave dielectric properties of ceramics. All these above results prove that the microstructure of Li3Mg2NbO6 ceramics is sensitive to the sintering temperature and the Li3Mg2NbO6 ceramics with 0.5 wt.% ZBS additives could be well densified at 925 °C and display a harmony grain distribution.
The bulk density, εr value and Q × f value of Li3Mg2NbO6 + xwt.%ZBS (x = 0.1–3.0) ceramics sintered at different temperatures are shown in Fig. 3a–c respectively. It is easy to find that the bulk densities of all compositions gradually increase with increasing ZBS content and reach the maximum value at their respective sintering temperature, the maximum bulk density(3.5222 g/cm3) is obtained in Li3Mg2NbO6 ceramics doped with 1.0 wt.% ZBS additives sintered at 950 °C for 4 h. Beside of these, we can see that the optimum sintering temperature reveals a decreasing variation trend with increasing ZBS content, but excessive additives could reduce the sintering temperature followed by an apparently decrease in bulk density. The above results confirm that appropriate amount of ZBS additives could effectively promote the densification and reduce the sintering temperature of Li3Mg2NbO6 ceramics. In general, the εr value is mainly dependent on the density, dielectric polarizability and structural characteristics such as distortion, tilting, and rattling spaces of oxygen octahedron in the unit cell [8, 16, 17]. In this paper, the εr value is mainly determined by the bulk density of the sample, since the variation trends of εr value are consistent with that of bulk density, the maximum εr value of 15.50 is appeared in the 1.0 wt.% ZBS added specimen sintered at 950 °C. Figure 3c shows the Q × f value of Li3Mg2NbO6 ceramics with various ZBS contents sintered at different temperatures. It is generally known that the quality factors mainly rely on the intrinsic and extrinsic losses, the intrinsic losses are related with the lattice anharmonicity [18–22], while the extrinsic losses are usually controlled by the porosity, second phase, impurity and grain size [23–28]. In present work, all the specimen are detected that no secondary phase and no impurity arise, as shown in Fig. 1. The Q × f values exhibit a similar variation trend with that of bulk density, demonstrating that the quality factors are mainly determined by the bulk density. The Q × f value increase to the maximum value initially, then gradually decrease with increasing ZBS content and sintering temperature. The increase in Q × f value could be due to the densification improvement benefiting from ZBS additives as shown in Fig. 2b, while the decrement of Q × f value is correlated with the relatively low bulk density and inferior microstructure as shown in Fig. 2e. The maximum Q × f value of 73,987 GHz is obtained in the sample doped with 0.5 wt.% ZBS additives sintered at 925 °C for 4 h. However, as x exceeds to 1.0, the Q × f values of specimen sintered at 900–950 °C suddenly decrease although bulk densities are higher compared to specimens with 0.5 wt.% ZBS. According to the SEM micrographs, the average grain size in the x = 1.0 specimen sintered at 900–950 °C is much smaller than that in the x = 0.5 specimen, and irregular grain growths are observed in Fig. 2c, i. So that, the decrease in Q × f value with high bulk density could be due to the relatively smaller grain size and inhomogeneous microstructure, which would result in a material with a higher dielectric loss and diminish the Q × f value [29]. Otherwise, these compositions with 0.5 wt.% and 1.0 wt.% ZBS have a high and similar bulk density. Consequently, the grain morphology factors have a more significant effect on the quality factor of Li3Mg2NbO6 ceramics while the density factors may be eliminated.
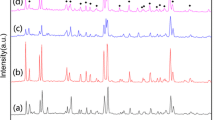
For chemical compatibility tests, the 0.5 wt.% ZBS doped Li3Mg2NbO6 ceramic sheet and Ag powders are co-fired at 925 °C for 4 h and analyzed to detect interactions between the low-fired samples and Ag powders. The corresponding XRD patterns is shown in Fig. 4 and it presents that no chemical reaction to form new phase after co-firing. In addition, The SEM image is used to evaluate the compatibility, as shown in Fig. 5. From the above analyses, it is obvious that the Li3Mg2NbO6 ceramics with 0.5 wt.% ZBS have a pretty good chemical stability against Ag electrode under co-firing condition, which could be selected as a promising candidate material for LTCC application due to its low sintering temperature, excellent microwave dielectric properties, and superior compatibility with Ag electrode.
Table 1 shows the effect of ZBS additives on the τf value of sample sintered at 925 °C for 4 h. The τf value is the parameter that represents thermal stability of microwave device and indicates how much the resonant frequency drifts with changing temperature. In our work, the τf value presents a decreased tendency as ZBS content increases, and smaller τf values are obtained in the sample doped with 0.1 wt.% and 0.5 wt.% ZBS additives, while Yuan et al. [5] have reported that the τf value of Li3Mg2NbO6 ceramics sintered at 1250 °C for 2 h is −27.2 ppm/°C. In short, fairly good microwave dielectric properties of εr ~ 14.84, Q × f ~ 73,987 GHz, τf ~ −16.05 ppm/°C are obtained in Li3Mg2NbO6 ceramics doped with 0.5 wt.% ZBS additives when sintered at 925 °C for 4 h.
4 Conclusion
Effects of ZBS additives on sintering behavior and microwave dielectric properties of Li3Mg2NbO6 ceramics are investigated systematically. The ZBS doped Li3Mg2NbO6 ceramics are obtained through a conventional solid state method and single phase with orthorhombic structure is performed in XRD patterns. The microwave dielectric properties of composite ceramics could be adjusted by changing the ZBS amounts. Meanwhile, the sintering temperature could be effectively decreased to below 961 °C by using small amount of ZBS additives. Especially, the 0.5 wt.% ZBS modified Li3Mg2NbO6 ceramics sintered at 925 °C present excellent microwave dielectric properties of εr ~ 14.84, Q × f ~ 73,987 GHz, τf ~ −16.05 ppm/°C. In addition, the Li3Mg2NbO6 ceramics with 0.5 wt.% ZBS have a good chemical compatibility with Ag electrode, which could be a promising material for LTCC application.
References
P. Zhang, Y. Wang, J. Liu, Z.K. Song, Y.M. Han, L.X. Li, Mater. Lett. 123, 195–197 (2014)
H.D. Xie, C. Chen, R. Tian, H.H. Xi, Mater. Lett. 162, 91–93 (2016)
S.P. Wu, D.F. Chen, C. Jiang, Y.X. Mei, Q. Ma, Mater. Lett. 91, 239–241 (2013)
J.J. Bian, D.W. Kim, K.S. Hong, Mater. Lett. 59, 257–260 (2005)
L.L. Yuan, J.J. Bian, Ferroelectrics 387, 123–129 (2009)
H.T. Wu, E.S. Kim, J. Alloy. Compd. 669, 134–140 (2016)
Y.G. Zhao, P. Zhang, J. Alloy. Compd. 658, 744–748 (2016)
T.W. Zhang, R.Z. Zuo, Ceram. Int. 40, 15677–15684 (2014)
T.W. Zhang, R.Z. Zuo, C. Zhang, Mater. Rev. 68, 109–114 (2015)
P. Zhang, J.W. Liao, Y.G. Zhao et al., J. Mater. Sci. Mater. Electron. (2016). doi:10.1007/s10854-016-5575-1
J.X. Tong, B. Zhang, W. Huang, H. Yang, Mater. Lett. 95, 168–171 (2013)
X.P. Lu, Y. Zheng, Z.W. Dong, Q. Huang, Mater. Lett. 131, 1–4 (2014)
X.P. Lv, Y. Zheng, B. Zhou, Z.W. Dong, P. Cheng, Mater. Lett. 91, 217–219 (2013)
W.E. Courtney, IEEE Trans. Microw. Theory Tech. 18, 476–485 (1970)
W.S. Kim, T.H. Kim, E.S. Kim et al. Jpn. J. Appl. Phys. 37, 5367–5371 (1998)
R.D. Shannon, G.R. Rossman, Am. Mineral 77, 94–100 (1992)
E.S. Kim, S.H. Kim, K.H. Yoon, J. Ceram. Soc. Jpn. 112, S1645–S1649 (2004)
C.L. Huang, S.H. Liu, J. Am. Ceram. Soc. 91, 3428–3430 (2008)
Q.W. Liao, L.X. Li, X. Ding, X. Ren, J. Am. Ceram. Soc. 95, 1501–1503 (2012)
C.L. Huang, J.Y. Chen, J. Am. Ceram. Soc. 92, 675–678 (2009)
S.J. Penn, N.M. Alford, A. Templeton, X.R. Wang, M.S. Xu, M. Reece, K. Schrapel, J. Am. Ceram. Soc. 80, 1885–1888 (1997)
C.L. Huang, C.H. Su, C.M. Chang, J. Am. Ceram. Soc. 94, 4146–4149 (2011)
H.T. Kim, S.H. Kim, S. Nahm, J.D. Byun, Y. Kim, J. Am. Ceram. Soc. 82, 3043–3048 (1999)
Y.C. Zhang, L.T. Li, Z.X. Yue, Z.L. Gui, Mater. Sci. Eng. B 99, 282–285 (2003)
J.X. Tong, Q.L. Zhang, H. Yang, J.L. Zou, J. Am. Ceram. Soc. 90, 845–849 (2007)
I.S. Cho, D.W. Kim, J.R. Kim, K.S. Hong, Ceram. Int. 30, 1181–1185 (2004)
M.H. Kim, Y.H. Jeong, S. Nahm, H.T. Nahm, H.J. Lee, J. Eur. Ceram. Soc. 26, 2139–2142 (2006)
D.H. Kang, K.C. Nam, H.J. Cha, J. Eur. Ceram. Soc. 26, 2117–2121 (2006)
B.D. Silverman, Phys. Rev. 125, 1921–1930 (1962)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 61671323) and Key Laboratory of Advanced Ceramics and Machining Technology, Ministry of Education (Tianjin University).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, P., Liu, L., Zhao, Y. et al. Low temperature sintering and microwave dielectric properties of Li3Mg2NbO6 ceramics for LTCC application. J Mater Sci: Mater Electron 28, 5802–5806 (2017). https://doi.org/10.1007/s10854-016-6251-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6251-1