Abstract
Copper sulfide nanostructures with different morphology have been developed using wet chemical route. The product so obtained have been characterized using X-ray diffraction (XRD), Field emission scanning electron microscopy (FESEM), Ultraviolet visible spectroscopy (UV–Vis) and photoluminescence (PL) technique. Effect of reaction medium on the morphology of the obtained samples has been studied in detail. We hereby demonstrate that while the reaction medium used has no impact on the phase of the prepared sample, however it plays an important role in deciding the morphology. CuS nanoparticles, nanoflower and nanoflakes like structure have been prepared under optimized condition using facile method in small period of time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor chalcogenides made of cheap and abundant metal ions with appropriate band gap are currently an attractive field for the researchers [1]. Among various chalcogenides, copper sulfide with unique characteristic properties has been a potential candidate for number of applications [2]. The activity of copper sulfide has been shown to improved significantly if prepared on nanoscale with well defined morphology [3]. Copper sulfide can be present in different crystallographic phases. Among these different phases, ideal band gap of CuS makes it efficient solar energy absorber as it possess variable band gap which can be engineered to an ideal band gap of solar cells which make it efficient light absorbers for fabrication of solar cell [4]. Therefore it is desirable to develop a process that can produce CuS nanostructure with different controlled morphology. CuS structure with different morphologies have been prepared using different techniques [5]. However, still it is a big challenge to produce pure nanostructured CuS. In the present work, we report the preparation of CuS nanostructure with diverse morphology. Furthermore, by changing the reaction solvent from ethylene glycol to polyethylene glycol CuS with well define morphology has been obtained.
2 Experimental
2.1 Chemicals
Copper chloride dihydrate, zinc acetate dihydrate, sodium sulphide flakes (purified) and sodium thiosulfate pentahydrate were obtained from Sigma and Aldrich. Polyethylene glycol, ethylene glycol and cetyl trimethyl ammonium bromide (CTAB) were purchased from Spectrochem, India. All the chemicals were of analytical grade and were used without any further purification.
2.2 Synthesis
2.2.1 Copper sulfide nanoparticles (sample 1)
In typical experiment 0.2 moles of Na2S solution was prepared in distilled water. Solution of 1 gram CTAB in 10 ml distilled water along with 25 ml of ethylene glycol was added to Na2S solution. To this mixture 0.1 mol of CuCl2.2H2O solution was added drop wise with continuous stirring over a magnetic stirrer at room temperature. Black colored solution so obtained was kept stirred over magnetic stirrer for 5–10 min. Then the mixture was refluxed for 6 h and after being allowed to cool down to room temperature it was filtered washed with ethyl alcohol and dried under ambient conditions.
2.2.2 Zn doped copper sulfide nanoparticles (sample 2)
0.1232 moles of copper chloride solution, 0.011 mol of zinc acetate and 0.269 moles of sodium sulfide solution was prepared separately. Then a solution of 1 g CTAB in 10 ml distilled water along with 25 ml of ethylene glycol was added to sodium sulfide solution while stirring. Followed by that, copper chloride and zinc acetate solutions were added, simultaneously, drop wise to the solution CTAB and Na2S solution with continuous stirring over a magnetic stirrer at room temperature. Black coloured solution so obtained was kept stirred over magnetic stirrer for 5–10 min. Then the mixture was refluxed for 6 h at and after being allowed to cool down to room temperature it was filtered washed with acetone and dried.
2.2.3 Copper sulfide nanoflowers (sample 3)
0.06 mol of CuCl2 solution was prepared in 50 ml distilled water. To this solution 50 ml polyethylene glycol was added. In similar way 0.03 mol of sodium thiosulfate solution was prepared in water and polyethylene glycol mixture. Both the solutions were sonicated for a period of 15 min. Sodium thiosulfate solution was added drop wise to the solution of copper chloride with continuous stirring at room temperature, over a period of 45 min. After that the mixture was kept stirred over magnetic stirrer for 5–10 min. Then the mixture was refluxed for 90 min and after being allowed to cool down to room temperature, black solid sample obtained, was filtered washed with absolute alcohol and dried under ambient conditions.
2.2.4 Copper sulfide nanoflakes (sample 4)
0.06 mol of copper chloride solution was prepared in 50 ml distilled water and then 50 ml polyethylene glycol was added to it. Similarly, 0.03 mol of sodium thiosulfate solution was prepared in 50 ml distilled water and then 50 ml polyethylene glycol was added to it. Both the solutions were sonicated for a period of 15 min. Solution of 3.58 g of CTAB in 25 ml distilled water was added to copper chloride solution with continuous stirring at 45 °C for 15 min. Now, sodium thiosulfate solution was added drop wise to the solution mixture of copper chloride and CTAB with continuous stirring at room temperature, over a period of 45 min. After that the mixture was kept stirred over magnetic stirrer for 5–10 min. Then the mixture was refluxed for 90 min and after being allowed to cool down to room temperature it was filtered washed with absolute alcohol and dried under ambient conditions.
2.3 Characterization
The powder X-ray diffraction pattern (XRD) were recorded by using a Rigaku MiniFlex II desktop X-ray diffractometer using Cu Kα radiation (1.54 Å) with a step size of 2° per minute. 2θ ranges from 25 to 700. Morphological investigations were performed on a Field Emission Scanning Electron Microscope (FESEM), model NOVA NANOSEM-430 of COMFEI. The optical property of the obtained samples was studied using HACH DR-3900 UV–Vis spectrophotometer. The photoluminescence measurements were performed using HITACHI F-7000 spectrophotometer.
2.4 Results and discussion
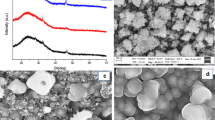
FESEM micrographs of synthesized samples are shown in Fig. 1. As can be seen from Fig. 1 that sample1 has nanoparticles like structure with average size being ~40 nm. Interestingly, after the addition of Zn to the prepared sample the morphology of the sample remains same with again average particle size of ~40 nm. Copper and Zinc has both very close ionic radii that may be the reason for the retention of the similar morphology even after addition of Zn to the prepared samples. Sample 3 whose synthesis process involves the sodium thiosulphate as the sulphur source consist of flower like structure with their petal’s thickness of ~10 nm and average distance between two petals being under 200 nm. Sample 4 where CTAB is involved along with the Na2S2O3 consists of nanoflakes, with thickness ~15 nm agglomerated randomly.
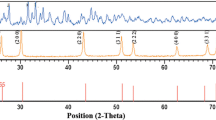
Figure 2 shows the XRD patterns of sample 1, 2, 3 and 4. Diffraction pattern reveals that the synthesized samples are polycrystalline in nature. It also confirmed the formation of hexagonal covellite phase, which matches well with the standard (JCPDS card no. 06-0464), having a = b = 3.792 Å and c = 16.34 Å as lattice parameters [6, 7]. As sample 2 is Zn doped CuS, i.e. Zn2+ replaces some of the Cu2+ in the lattice site, and the ionic radii of Cu2+ and Zn2+ is nearly the same therefore only minor (negligible) shift in the peak position has been observed; and as no other peaks corresponding to ZnS or ZnO is found. It implies that Zn has been successfully doped in CuS. XRD pattern reveals that sample 3 and sample 4 are copper sulphide as most of the major peaks are matched well to the standard JCPDS card no. 06-0464 corresponds to hexagonal covellite phase with lattice parameters a = b = 3.792 Å, c = 16.34 Å. However both samples 3 and 4 contain Atacamite (copper chloride hydroxide) as a minor impurity phase. Efforts were made to remove these impurities by heat treatment followed by washing. XRD pattern of the sample 4, dried at a 45–55 °C, confirmed that impurity can be removed by heat treatment followed by washing. These results further support the fact that the reaction medium has no great impact on the phase of the obtained samples.
Figure 3 shows the absorbance vs wavelength obtained for all four samples. Absorption spectra of Sample 1 consist of two distinct broad features at ~450 nm and other in near IR region. While, sample 2, sample 3 and sample 4 possess a broad absorption band only in the near-IR region. These characteristic features are in agreement with the literature and can be attributed to the covellite (CuS). These are because of interband transition from valence states to unoccupied states. Figure 4 shows the bandgap plot of (αhυ)2 vs Eg (hν), Tauc’s plot, obtained by Tauc’s equation given by.
UV–visible absorbance spectra of copper sulfide nanoparticles (sample 1), Zn doped copper sulfide nanoparticles (sample 2), copper sulfide nanoflowers (sample 3) and copper sulfide nanoflakes (sample 4) recorded using HACH DR-3900 UV–Vis spectrophotometer with variation of wavelength from 250 to 950 nm
where α = (2.303 x A)/t, is absorption coefficient, K is a constant, h is Planck’s constant, ν is frequency and Eg is energy band gap. Now, band gap was calculated by extrapolating the linear region of (αhν)2 vs hν curve. Due to the presence of broad features, except for Sample 1, sharp transitions were not used to calculate the band gap therefore the maximum absorption position was used to determine the band gaps of different samples. As can be seen from Fig. 4 that Sample 1 shows the band gap of 2.021 eV while for sample 2, 3 and 4 very close band gap of 3.173, 3.136 and 3.211 eV obtained respectively. From these results it can be concluded that the doping as well as the morphology of the copper sulfide has significant impact on the band gap of the material obtained [8]. Further, these band gap values were in line with those reported in literature for synthesis of copper sulphide nanoparticles by various methods 0.13–3.4 eV [9, 10].
Because of its excellent photoconductive, photovoltaic and rectifying response under illumination the photoluminescence (PL) property of CuS is of great importance. PL spectra obtained for different samples at excitation wavelength of 320 nm is shown in Fig. 5. All the samples show two broad emission features, one major peak around 380–400 nm and another broad feature between 450 and 480 nm. The characteristic emission features obtained for the samples matches well with the literature report [11].
The solvents have been known to play important role in the synthesis of the chalcogenides. Alcohols such as ethylene gycol, polyethylene gycol, benzyl alcohol have been known to act as reducing or stabilizing agent in the formation of the copper sulphides. The above results show that the reaction medium used in the present work has no effect on the phase of the copper sulfides however it significantly affects the morphology. In synthesis of Sample 1, the presence of ethylene glycol and the CTAB results in the formation of well defined spherical nanoparticles. On replacing the ethylene glycol with the polyethylene glycol for Sample 4, nanoflakes like structure was obtained. This observation shows that the choice of alcohol is leading to the change in the morphology. Polyethylene glycol is known to have higher viscosity and surface hydroxyl group compared to the ethylene glycol. Nucleation and the self assembly of the nanoparticle will occur at different rates in presence of solvents with different viscosity [12]. For Sample 1, the stablising effect of ethylene glycol results in the formation of initial particles with spherical shape and much smaller in overall size. While for Sample 4, in presence of poly ethylene glycol, these nanoparticles aggregate and self assemble to form plate like structure. On obivating the CTAB from the reaction medium used for Sample 3, very well defined flower like structure was obtained. This may be due to the fact that presence only polyethylene glycol leads to better stabilizing effect thereby leading to the more ordered aggregation of nanoplates resulting in formation of flower like morphology [13].
In conclusion, in the present work optimum reaction conditions have been developed to form copper sulfide (CuS) nanostructures with different morphology. Synthesis has been done by facile wet chemical route using various precursors. XRD pattern revealed the presence of covellite phase. UV–visible and photoluminescence characteristics are in well accordance to the results mentioned in literature. FESEM micrographs show that samples of different shapes/morphology including nearly spherical particles, nano-flowers and nano-falkes were obtained. Different morphology so obtained can be attributed to the proper solubility, diffusivity and self assembly of the reactants under the reaction medium used. The synthetic strategy developed in present work will be a promising step in the development of inorganic structures as well as functional devices.
References
H. Zhou, W-C. Hsu, H-S. Duan, B. Bob, W. Yang, T-B. Song, C-J. Hsu, Y. Yang, CZTS nanocrystals: a promising approach for next generation thin film photovoltaics. Energy Environ. Sci. 6, 2822–2838 (2013). doi:10.1039/c3ee41627e.
M. Tanveer, C. Cao, I. Aslam, Z. Ali, F. Idrees, M. Tahir, W.S. Khan, F.K. Butt, A. Mahmood, Effect of morphologies of CuS upon the photo-catalytic degradation of organic dyes. RSC Adv. 4, 63447–63456 (2014). doi:10.1039/C4RA04940C
W. Xu, S. Zhu, Y. Liang, Z. Li, Z. Cui, X. Yang, A. Inoue, Nanoporous CuS with excellent photocatalytic property, Sci. Rep. Article number: 18125 (2015). doi:10.1038/srep1812
K.R. Nemade, S.A. Waghuley, Band gap engineering of CuS nanoparticles for artificial photosynthesis. Mater. Sci. Semicond. Process. 39, 781–785 (2015). 10.1016/j.mssp.2015.05.045.
M. Saranya, A.N. Grace, Hydrothermal synthesis of CuS nanostructures with different morphology. J. Nano Res. 18–19, 43–51 (2012)
Powder diffraction file, alphabetical index inorganic phases: JCPDS International Centre For Diffraction Data 1601, Park Lane Swarthmore, Pennsylvania 19081 USA set 6–10:87 (1984)
JCPDS-International Centre for Diffraction Data (ICDD) (1997–2016)
M. Saranya, C. Santhosh, R. Ramachandran, G.A. Nirmala (2014) Growth of CuS nanostructures by hydrothermal route and its optical properties. J. Nanotechnol. (2014). Article ID 321571. doi:10.1155/2014/321571.
R.S. Christy, T.T. Kumaran, Phase transition in Cus nanoparticles. J. Non Oxide Glass. 6, 13–22 (2014)
B.R. Sankapal, R.S. Mane, C.D. Lokhande, Preparation and characterization of Bi 2 Se 3 thin films deposited by successive ionic layer adsorption and reaction (SILAR) method. Mater. Chem. Phys. 63, 226 (2000)
P. Priyadharshini, R. Rajagopal, Synthesis and characterization of metal doped nanocrystalline copper sulphide. Int. J. Recent Sci. Res. 6: 3328–3331 (2015). http://www.recentscientific.com.
S.K. Goswami, J. Kim, K. Hong, E. Oh, Y. Yang, D. Yu, Photocurrent and photovoltaic characteristics of copper sulfide nanowires grown by a hydrothermal method. Mater. Lett. 133, 132–134 (2014). 10.1016/j.matlet.2014.06.173
H. Ke, W. Luo, G. Cheng, X. Tian, Z. Pi, Synthesis of flower-like CuS nanostructured microspheres using poly(ethylene glycol)200 as solvent. J. Nanosci. Nanotechnol. 10(11), 7770–7773 (2010). doi:10.1166/jnn.2010.2866
Acknowledgements
We are thankful to Mr. Gaurav Gupta, Mr Anup Khare and Mr. Mohd. Shafique, CSIR-AMPRI, Bhopal (MP), India for their help in performing FESEM and XRD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kushwah, R., Singh, A., Anshul, A. et al. Facile and controlled synthesis of copper sulfide nanostructures of varying morphology. J Mater Sci: Mater Electron 28, 5597–5602 (2017). https://doi.org/10.1007/s10854-016-6227-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6227-1