Abstract
Nanostructured metal (Sn, Cu and Ni) doped TiO2 was successfully synthesized by microwave irradiation method. Metal doped TiO2 was characterized by X-ray diffraction (XRD), Scanning electron microscope (SEM), Fourier transform infrared spectroscopy (FTIR) and UV–Vis spectroscopy (UV–Vis). The XRD pattern confirms the formation of monoclinic phase TiO2 and metal doped TiO2 samples. SEM images show the attractive morphologies for metal doped TiO2 nanopowders. The photocatalytic activity of the synthesized sample was also studied by the decomposition of methylene blue dye under UV light irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photocatalytic effect at the surface of titanium dioxide has been attracting much interest to examine their useful applications for self cleaning of tiles, glasses, and windows, air and water purification [1]. Water pollution is one of the major contaminations in the world. Several treatments were used in order to solve this problem. Among them photocatalytic decomposition is one of the best method for waste water treatement. Since, the first discovery of photocatalytic splitting of water with TiO2 under ultra violet (UV) light irradiation by Fujishima and Honda [2], the semiconductor metal oxides (e.g..TiO2, CdS, ZnS, Fe2O3 and ZnO) are attracting much attention for use in photocatalysis [3–16]. Titanium dioxide is one of the best semiconductor oxides due to its cost effective, eco-friendly nature, photostability, chemical inertness and high stability [17–23]. It has been potential material owing to its environmental applications such as photon-to-electric energy conversion devices or water-splitting applications and pollutant dissociation [24–26]. TiO2 exists in three different crystalline polymorphic phases such as anatase, rutile and brookite [27]. The rutile and anatase phases have an energy band gap of 3.0–3.2 eV and hence it can absorb only <5% of the solar radiation that reaches the earth’s surface [28]. For this function, doping or combining TiO2 with different non-metal or transition metal ions has been initiated. In this work, Sn, Cu and Ni have been selected for doping with TiO2. Tin is nontoxic, abundant, and inexpensive and has relatively high chemical stability. Sn-doped titania also illustrated improved day-light activity and it has a wide band gap of 3.8 eV [29, 30]. It is highly enviable to manipulate size distribution and design, surface chemistry and chemical composition of tin oxide nanocrystals reaction to visible-light, which accounts for 43% of the entire sunlight [31]. Cu is a relatively abundant and low cost metal [32] and it nearly inhibits electron–hole recombination to improve the catalyst. Some reports exposed that Cu-doped TiO2 nanoparticles could be fabricated via a vapor-phase syntheses (e.g., evaporation condensation and sputtering) or liquid-phase syntheses (e.g., sol–gel and impregnation) [33]. Nickel has been established to be a capable transition metal for increasing the photocatalytic activity when it is doped with TiO2. Ni-doped mesoporous TiO2 catalyst played a major role in controlling the morphology and thermal stability of mesoporous photocatalysts [34, 35]. Transition metal doped titanium dioxides were synthesized by various methods such as sol–gel method [36], anodization method [37], electrospinning method [38], hydrothermal and solvothermal method [39], spin-coating technique [40] etc. These methods are tedious in contrast to the microwave irradiation method. The microwave irradiation method has sparked much attention owing to its simplicity, logically low cost, short period and likelihood of huge scale construction [41, 42]. In the present work, TiO2 and metal doped TiO2 nanomaterials were synthesized by microwave irradiation method and subsequently characterized the materials using several advanced techniques. The photocatalytic performance of the synthesized nano-sized TiO2 and metal doped TiO2 nanopowders was also investigated by methylene blue dye under the visible light irradiation.
2 Materials and methods
2.1 Materials
Commercially available titanium oxychloride (TiOCl2), stannous chloride dihydrate, copper acetate monohydrate, nickel(II) acetate tetrahydrate, sodium hydroxide (NaOH), methylene blue, deionized water (DD) were used as received.
2.2 Experimental methods
Metal doped TiO2 powders were prepared by microwave irradiation method using the following procedure. A mixture of 1.6 M titanium oxychloride (TiOCl2) was prepared using deionized water as solvent and stirred at room temperature. Titanium precursor was added to the transition metals (0.1 M stannous chloride dihydrate, 0.1 M copper acetate monohydrate, 0.1 M nickel(II) acetate tetrahydrate) after stirring for 30 min. Sodium hydroxide (NaOH) solution was added dropwise until the pH of the solution reached 12 and maintained at 90 °C. After 1 h vigorous stirring, solution was obtained. Same procedure was followed and then added 1.6 M titanium oxychloride and NaOH without transition metals. The microwave irradiation was passed through the solution with the frequency of 2.45 GHz with power of 160 W for 30 min. Subsequently TiO2 and metal doped TiO2 powders were formed.
2.3 Photocatalytic measurement
The photocatalytic activities of the synthesized TiO2 and metal doped TiO2 nanomaterials were evaluated by the degradation of methylene blue under a 6-W UV lamp equipped with a cutoff filter (λ > 254 nm). The illumination intensity used for the photodegradation of dye was 6 W UV lamp—Philips. To keep the photocatalytic reaction at room temperature, the reaction beaker was kept in a cooling water system. An amount of 0.1 g of photocatalyst was suspended in a 200 mL aqueous methylene blue (MB) dye solution (10 mg/L). Prior to the illumination, the suspensions were ultra-sonicated for 5 min under dark condition for 1 h to ensure an adsorption–desorption equilibrium of the MB dye was adsorbed to saturation level on the surface of catalysts. The effect of concentration of the catalysts on the degradation of dye was also investigated.
2.4 Characterization
The structural property of metal doped TiO2 was studied by X-ray diffraction (XRD) using Cu-Kα (λ = 0.154 nm) radiation source (X’pert Pro PANalytical) over a 2θ scan range of 10–80°. Morphological studies of the samples were examined by Scanning Electron Microscope (SEM; JEOL JCM-6000, 15 KV). The quality and composition of the nanoparticles were characterized by Perkin Elmer-Fourier transform infrared (FT-IR) spectrometer in the range of 400–4000 cm−1 in KBr pellets. The MB dye degradation was measured by UV–Vis spectrophotometer (SCHIMADZU UV-1800) in the wavelength range of 400–800 nm.
3 Results and discussion
3.1 X-ray diffraction (XRD)
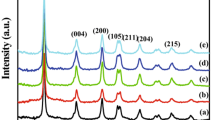
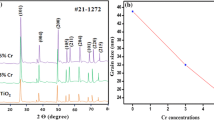
The as-prepared TiO2 and metal (Sn, Cu and Ni) doped TiO2 powder was found to be crystalline nature. The XRD pattern was also measured for the samples which were subjected to microwave irradiation for 30 min. Figure 1a–d shows the XRD pattern of the synthesized TiO2 and metal (Sn, Cu and Ni) doped TiO2 powders. XRD pattern of TiO2 powder is well indexed to the standard pattern of monoclinic phase which is in accordance with the JCPDS card no.481278. The sharper peaks clearly indicate the presence of TiO2. The pure TiO2 peak was large compared to those of metal doped TiO2 samples. The X-ray powder diffraction patterns were also used to determine the crystallite size of all the prepared samples. The crystallite size D was determined from the broadening of the peaks by Scherrer’s formula.
where K is the shape factor (0.9), λ is the wavelength, β is the width of the peak at half the maximum intensity (FWHM) after subtraction of instrumental noise, and θ is the diffraction angle. The estimation shows that the mean crystallite size of TiO2 and metal (Sn, Cu and Ni) doped TiO2 are in the range of 57 nm and 20 nm, 27 nm and 30 nm respectively.
3.2 SEM with EDX analysis
SEM images of TiO2 and metal (Sn, Cu and Ni) doped TiO2 powders are shown in Fig. 2. The SEM image of TiO2 and metal (Sn, Cu and Ni) doped TiO2 powder indicates the presence of particles of different shapes and sizes. Figure 2a display the SEM image of pure TiO2 sample and it clearly indicates the aggregation of particles. The addition of various doping materials such as Sn, Cu and Ni influences the surface morphology of the TiO2 powder. Figure 2b shows the SEM image of Sn-TiO2 powder and it clearly evinces the formation of nanoparticle. Figure 2c shows the image of Cu-TiO2 sample and it clearly point out the formation of flower like structure. Careful observation of SEM image of Ni-TiO2 sample shows the presence of nanoplate morphology (Fig. 2d). Figure 3a–c show the EDX spectra of TiO2, Sn doped TiO2 and Ni doped TiO2 and the EDX spectra confirms the presence of Ti, Sn, O and Ni elements, respectively.
3.3 Fourier transform infrared spectroscopy (FTIR)
Figure 4 shows FTIR spectra of TiO2 and metal (Sn, Cu and Ni) doped TiO2 samples in the range of 400–4000 cm−1 at room temperature. The bands observed in the range of 400–800 cm−1 are mainly ascribed to Ti–O and Ti–O–O bonds. Since Ti–O bond is shorter than Sn–O, Cu–O and Ni–O bonds therefore, the doping of metal in TiO2 may lead to a shift of the wavenumber of Ti–O lattice vibration [43–45]. The bands observed in the range of 1101 and 1026 cm−1 are present in all the samples which indicate Ti–O–Ti vibrations [34]. The band at 1445 cm−1 in TiO2 has been attributed to stretching vibration of Ti–O–Ti group which is shifted to 1409 cm−1.This may be due to the doping metals in of TiO2 [46]. The stretching vibration of O–H and bending vibration of the adsorbed water molecules are also present at 1645 cm−1 in TiO2 and Sn doped TiO2. Then Cu and Ni doped into TiO2 the peak shifted to 1564 cm−1this may be due to the doping of Cu and Ni [34]. The peaks observed at 2922 cm−1 corresponds to residue of bioorganic substances [47]. The presence of an intense H2O band at 3430, 3444 and 3452 cm−1 confirms the presence of hydroxyl groups or adsorbed water [30].
3.4 Ultraviolet visible spectroscopy (UV–Vis)
Figure 5a–d shows UV–Vis spectra of methylene blue dye in the presence of TiO2 and metal (Sn, Cu and Ni) doped TiO2 sample under UV light. Microwave treatment for 30 min under the irradiation of light above 254 nm was performed for the sample. The peak intensity of methylene blue dye absorption was found at 665 nm and the intensity of the methylene blue absorption decreased with an increase of the reaction time from 0 to 3 h [48].
3.5 Photocatalytic activity test
Photocatalytic activity of the synthesized TiO2 and metal (Sn, Cu and Ni) doped TiO2 samples was performed by the degradation of Methylene Blue (MB) in aqueous solution under UV light irradiation. MB shows a maximum absorption at about 665 nm. Figure 6a–d shows the decomposition of MB in aqueous solution in the presence of TiO2 and metal (Sn, Cu and Ni) doped TiO2 which was prepared by the microwave treatment. The MB degradation study was carried out under UV light irradiation (λ > 254 nm). The degradation efficiency of the samples was defined as C/C0, where C and C0 stood for the remnants and initial concentration of MB. The degradation efficiency of MB was calculated as 92, 85, 60 and 54% for the samples TiO2, Sn–TiO2, Cu–TiO2 and Ni–TiO2, respectively. The activity of the TiO2 photocatalytic efficiency was high compared to metal doped TiO2.This is due to the change of number of active sites, surface group type (or) acid-base properties in the metal (Sn, Cu and Ni) doped TiO2 samples which depends on the ionic radius, charge and low diffusion length. This also serves as a trap for the electron produced under irradiation [49].
4 Conclusions
The pure TiO2 and metal (Sn, Cu and Ni) doped TiO2 photocatalyst was successfully synthesized by microwave irradiation method. The XRD pattern confirmed the formation of monoclinic TiO2 and metal (Sn, Cu and Ni) doped TiO2. SEM image revealed the morphology of the prepared samples. FTIR studies confirmed the functional groups. The prepared pure TiO2 and metal (Sn, Cu and Ni) doped TiO2 photocatalyst successfully decomposed the methylene blue dye under UV light irradiation.
References
A. Zaleska, Recent Pat. Eng. 2, 157–164 (2008)
Z. Zhang, Y. Zhou, Y. Zhang, X. Sheng, S. Zhou, S. Xiang, Appl. Surf. Sci. 286, 344–350 (2013)
B. Pant, H.R. Pant, N.A.M. Barakat, M. Park, K. Jeon, Y. Choi, H.Y. Kim, Ceram. Int. 39, 7029–7035 (2013)
S. Karuppuchamy, M. Iwasaki, H. Minoura, Vacuum 81, 708–712 (2007)
T. Furukawa, H. Matsui, H. Hasegawa, S. Karuppuchamy, M. Yoshihara, Solid State Commun. 142, 99–103 (2007)
S. Yamamoto, H. Matsui, S. Ishiyama, S. Karuppuchamy, M. Yoshihara, Mater. Sci. Eng. B 135, 120–124 (2006)
H. Matsui, T. Kawahara, R. Kudo, M. Uda, S. Karuppuchamy, M. Yoshihara, J. Alloys Compd. 462, 20–23 (2008)
H. Matsui, S. Karuppuchamy, J. Yamaguchi, M. Yoshihara, J. Photochem. Photobiol. A Chem. 189, 280–285 (2007)
H. Matsui, T. Kuroda, K. Otsuki, K. Yokoyama, T. Kawahara, S. Karuppuchamy, M. Yoshihara, Tanso 222, 114–117 (2006)
M. Thamima, S. Karuppuchamy, Adv. Sci. Eng. Med. 7, 18–25 (2015)
T. Oekermann, S. Karuppuchamy, T. Yoshida, D. Schlettwein, D. Wohrle, H. Minoura, J. Electrochem. Soc. 151, 62–68 (2004)
S. Karuppuchamy, S. Ito, Vacuum 82, 547–550 (2008)
H. Miyazaki, H. Matsui, T. Kuwamoto, S. Ito, S. Karuppuchamy, M. Yoshihara, Micropor. Mesopor. Mater. 118, 518–522 (2009)
H. Miyazaki, H. Matsui, Y. Kita, S. Karuppuchamy, S. Ito, M. Yoshihara, Curr. Appl. Phys. 9, 155–160 (2009)
R. Dhilip Kumar, S. Karuppuchamy, J. Mater. Sci. Mater. Electron. 26, 3256–3261 (2015)
H. Matsui, N. Bandou, S. Karuppuchamy, M.A. Hassan, M. Yoshihara, Ceram. Int. 38, 1605–1610 (2012)
A.M.H. Milad, L.J. Minggu, M.B. Kassim, W.R.W. Daud, Ceram. Int. 39, 3731–3739 (2013)
S. Karuppuchamy, N. Suzuki, S. Ito, T. Endo, Curr. Appl. Phys. 9, 243–248 (2009)
K. Santhi, P. Manikandan, C. Rani, S. Karuppuchamy, Appl. Nanosci. 5, 373–378 (2015)
N. Suzuki, S. Karuppuchamy, S. Ito, J. Appl. Electrochem. 39, 141–146 (2009)
H. Matsui, T. Okajima, S. Karuppuchamy, M. Yoshihara, J. Alloy. Compd. 468, 27–32 (2009)
H. Matsui, S. Nagano, S. Karuppuchamy, M. Yoshihara, Curr. Appl. Phys. 9, 561–566 (2009)
H. Matsui, Y. Saitou, S. Karuppuchamy, M.A. Hassan, M. Yoshihara, J. Alloy. Compd. 538, 177–182 (2012)
S. Lee, Y. Lee, D.H. Kim, J.H. Moon, Appl. Mater. Interfaces 5, 12526–12532 (2013)
S. Karuppuchamy, Y. Andou, T. Endo, Appl. Nanosci. 3, 291–293 (2013)
N. Okada, S. Karuppuchamy, M. Kurihara, Chem. Lett. 34, 16–17 (2005)
K. Palanivelu, J.S. Im, Y.S. Lee, Carbon Sci. 8, 214–224 (2007)
W.I. Nawawi, M.A. Nawi, J. Mol Cata. A Chem. 374–375, 39–45 (2013)
H. Sayılkan, Appl. Catal. A General 319, 230–236 (2007)
V.B.R. Boppana, R.F. Lobo, ACS Catal. 1, 923–928 (2011)
C.M. Fan, Y. Peng, Q. Zhu, L. Lin, R.X. Wang, A.W. Xu, J. Phys. Chem. C 1172, 24157–24166 (2013)
T.D. Pham, B.K. Lee, Appl. Surf. Sci. 296, 15–23 (2014)
C.Y. Tsai, H.C. Hsi, T.H. Kuo, Y.M. Chang, J.H. Liou, Aerosol. Air Qual. Res. 13, 639–648 (2013)
A.K. Tripathi, M.C. Mathpal, P. Kumar, V. Agrahari, M.K. Singh, S.K. Mishra, M.M. Ahmad, Adv. A. Agarwal. Mater. Lett. 6, 201–208 (2015)
K.J. Salem, M.T. Hammad, R.R. Harrison, J. Mater. Sci. Mater. Electron., 24, 1670–1676 (2013)
S. Cizauskaite, V. Reichlova, G. Nenartaviciene, A. Beganskiene, J. Pinkas, A. Kareiva, Mater. Sci. 25, 3 (2007)
B. Samran, P. Krongkitsiri, S. Pimmongkol, S. Budngam, U. Tipparach, Adv. Mater. Res. 802, 104–108 (2013)
J. Vonch, A. Yarin, C.M. Megaridis, J. Undergraduate Res 1, 1 (2007)
S. Komarneni, Curr. Sci. 85, 12 (2003)
Y.Y. Huang, K.S. Chou, Ceram. Int. 29, 485–493 (2003)
R. Dhilip Kumar, S. Karuppuchamy, J. Mater. Sci. Mater. Electron. 26, 6439–6443 (2015)
R. Dhilip Kumar, S. Karuppuchamy, Ceram. Int. 40, 12397–12402 (2014)
M.R. Mahmoudian, W.J. Basirun, Y. Alias, M. Ebadi, Appl. Surf. Sci. 257, 8317–8325 (2011)
N. Wongpisutpaisan, N. Vittayakorn, A. Ruangphanit, W. Pecharapa, Sains Malaysiana 42, 175–181 (2013)
G. Viruthagiri, P. Praveen, S. Mugundan, E. Gopinathan, Indian J. Adv. Chem. Sci. 1, 132–138 (2013)
E.H. Faria, A.L. Marçal, E.J. Nassar, K.J. Ciuffi, P.S. Calefi, Mater. Res. 10, 413–417 (2007)
P. Dhandapani, S. Maruthamuthu, G. Rajagopal, J. Photochem. Photobiol. B Biol. 110, 43–49 (2012)
J. Maragatha, K. Jothivenkatachalam, S. Karuppuchamy, J. Mater. Sci. Mater. Electron. 27, 9233–9239 (2016)
S.N.R. Inturi, T. Boningari, M. Suidan, P.G. Smirniotis, Appl. Cata. B Environ. 144, 333–342 (2014)
Acknowledgements
The authors are grateful to the Department of physics and chemistry, Alagappa University for providing the XRD, SEM and FTIR characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maragatha, J., Rajendran, S., Endo, T. et al. Microwave synthesis of metal doped TiO2 for photocatalytic applications. J Mater Sci: Mater Electron 28, 5281–5287 (2017). https://doi.org/10.1007/s10854-016-6185-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6185-7