Abstract
The TiO2 NPs is a common used multifunctional material, because of its wide variety of application. While numerous natural mixes can be deteriorated and mineralized by oxidation and diminishment forms alluring on the surface of TiO2. A novel photocatalytic TiO2/SiO2–CdS nanocomposite were successfully preparation of sol–gel method and hydrothermal method. The developed nanocomposite was characterized by X-ray diffraction (XRD), Raman spectroscopy; UV–Visible Diffuse reflectance spectroscopy (DRS), Field emission scanning electron microscope (FE-SEM), Energy dispersive X-ray spectroscopy (EDAX) and Fourier transform infrared spectroscopy (FT-IR). The TiO2/SiO2–CdS nanocomposite has been used in the photocatalytic degradation of methylene blue (MB) dye under direct sunlight measured by UV–Visible spectroscopy. CdS NPs homogeneous surface exposure on the surface of TiO2/SiO2 nanocomposite was highly enhancing photocatalytic dye degradation of methylene blue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The previous couple of years, expanding new renewable viability wellspring of earth debased for brought on by the blazing remnant energies has prompted the looking for the new renewable vitality source. Consequently the hydrogen vitality establishment is another altered foundation is a new modified energy resource and potentially applicable for storable clean energy technology, water sanitation innovation [1, 2]. The photocatalytic splitting of water by TiO2 electrodes was published in 1972 by Honda and Fujishima, was developed in photocatalysis have explained by potential application in areas reach to convert solar energy, recycling polluted in water energy resource [3–5].
The semiconductor material TiO2 NPs firmly oxidizing power and concentrated on natural application, such as solar cell application, water technology, sensors, biosensors water purification techniques and catalytic dye degradation under the light irradiation [6–8]. Among the TiO2 semiconductor material as towering energy band gap (3.0 eV for the rutile phase and 3.2 eV for the anatase phase). The TiO2 is one of the most promising and challenge material low toxicity, less chemical inertness and high photo chemically stable [9–11]. Attributable to the TiO2 nanoparticles surface modified for nanorods [12, 13], nanotube [14, 15], nanowires and nano fiber have highly synergist the catalytic action [16–20]. At the point when a few techniques has been created the photo catalytic movement of TiO2, for example expanding the permeable way of TiO2 and producing the blemish of destination, surface change of metal, nonmetal, polymer expansion of semiconductor metal oxides [21–25]. The TiO2 on various semiconductors porous nature material such as the SiO2, ZnO, carbon and CdS doped was used to increase the adsorption capacity of organic dye molecules.
In this paper surface modified of TiO2 NPs which are composites with SiO2 nanoparticles in addition of decorated with CdS NPs using a hydrothermal method dispersed on the surface of TiO2. As per this TiO2/SiO2–CdS nanocomposite for further investigation was incredible consideration of increasing surface area and adsorptive of photocatalytic dye degradation. Among the TiO2/SiO2–CdS nanocomposite were suitable dye selected in methylene blue dye reaction carried out in direct sunlight, the dye degradation shortens the time period of the optimum level.
2 Materials and methods
2.1 Materials
Every one of the chemicals acquired from systematic evaluation in Merck chemicals Mumbai, India, Cadmium nitrate (CdNO3·4H2O, AR 99.99%), Sodium sulphide (Na2S·9H2O, AR 99.97%). Absolute methanol (MeOH), ethanol (EtOH), ammonium hydroxide (NH4OH, 98.99%), sodium hydroxide (NaOH, 99.99%) and hydrochloric acid (HCl, 99.99%) were received from Merck Chemicals. The various chemicals utilazied as part of this work were systamatic evaluation. Double distilled water was used for the preparation of aqueous solutions.
2.2 Synthesis of TiO2/SiO2–CdS nanocomposite
TiO2/SiO2 nanocomposite synthesized was according to our previous work [26]. The CdS NPs was prepared by following the reported article [27–29]. The three component of TiO2/SiO2–CdS nanocomposite weight ratio of (TiO2/SiO2–CdS = 0.250: 0.125: 0.075 g). Firstly, 0.5 mmol L−1 of cadmium nitrate Cd(NO3)2·4H2O, 1.5 mmol L−1 of Na2S·9H2O and 0.03 mmol L−1 of glutathione. The solvent was composed of 20 ml water and 20 ml absolute ethyl alcohol. 1 g of TiO2/SiO2 nanocomposite was placed in a Teflon-lined stainless-autoclave, which was then sealed and maintained at 453 K for 3.5 h. After the reaction, the resultant sample was washed with deionized water and absolute ethanol three times respectively and dried at 60 °C overnight and collect the TiO2/SiO2–CdS nanocomposite (Scheme 1 ).
2.3 Characterization
The X- ray diffraction (XRD) analysis of the TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite was recorded in Scintag-XDS-2000 spectrometer, with Cu Kα radiation under applied voltage of 40 KV and current of 30 mA. UV–Visible diffuse reflectance spectroscopy (DRS) of the nanocomposite was taken using ISR-2200 DRS accessory of UV–Vis spectrophotometer (UV-2550, Shimadzu). The TiO2/SiO2–CdS nanocomposite evolution of the surface morphology was analyzed by field emission scanning electron microscope (FE-SEM, JSM-7600F Japan) and energy dispersive X-ray spectroscopy (EDAX) was used to find out its elemental composition. Absorption spectrum of TiO2, TiO2/SiO2 and TiO2/SiO2–CdS catalyst was recorded using UV–Vis spectrophotometer (UV-2550, Shimadzu). Raman spectra analysis TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite was recorded in JASCO NRS-3100 laser Raman spectroscopy. FT-IR spectra analysis of the functional group was performed (FT-IR Shimadzu-650) using KBr pellet disk.
2.4 Photocatalytic measurement
Photocatalytic activities of TiO2 NPs, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite were evaluated by the degradation of MB dye under direct sunlight irradiation. The reaction cell was placed in a sealed black box with an opening on the top to provide visible-light irradiation. In a typical process, 100 mg of TiO2 NPs, TiO2/SiO2 and TiO2/SiO2–CdS photocatalyst was separately added in 100 ml of MB dye with a concentration of 10 mg/L. After being composite material is introduced in to an ultrasonic bath for 5 min, the solution was stirred for 30 min in the dark to reach the adsorption/desorption equilibrium between the catalyst on the surface. Then, the suspension was exposed to the direct sunlight irradiation. The samples were collected at given regular time intervals and the dye concentration was measured by using UV–Vis spectroscopy.
3 Results and discussion
3.1 X-ray diffraction
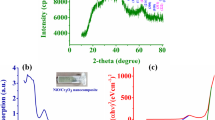
The XRD pattern of TiO2 NPs, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite for further examined crystalline nature has been demonstrated in Fig. 1. It’s due to represent the peaks at 2θ values, 25.11, 38.38, 48.48, 53.61, 55.64, and 62.80 corresponding to the (1 0 1), (0 0 4), (2 0 0), (1 0 5), (2 1 1) and (2 0 4) crystal plane of TiO2 JCPDS-card No: 02-1120. In addition of amorphous peak at 2θ value, 28.32 and crystal plane of (1 1 0) SiO2 was detected. They expect for the diffraction peaks attributed to the CdS NPs at 2θ values 26.5 and 43.7, the crystal plane of (1 1 1) and (2 2 0) (JCPDS-card No: 65-8193) were found to be a CdS present in TiO2/SiO2–CdS nanocomposite [30–33]. This could be very useful of nanocatalyst highly crystalline nature and enhancing the photocatalytic activity. The crystalline size is calculated using Scherer Eq. 1.
where λ wavelength of X-ray and β is a full width and the half maximum θ is the Bragg angle K is the shape of factor. The shape of TiO2 and TiO2/SiO2–CdS nanocomposite typical crystal size value is 16.4 and 27.9 nm respectively calculated from the most intense peak is available in XRD pattern. The sharp peak of TiO2/SiO2–CdS nanocomposite is extremely crystalline in nature.
3.2 Raman spectra analysis
Further investigate for Raman spectra testify the crystalline phase and study the effect of CdS structure on the TiO2/SiO2 nanocomposite. The Raman spectra were carried out TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite is shown in the Fig. 2. There are three main peaks at 425, 551 and 665 cm−1, Raman active mode for with assigned to the symmetries B1g, A1g and Eg source of the titania anatase phase respectively [34, 35]. In addiation of two peaks of CdS NPs approximately present at 288 and 588 cm−1 were observed. It’s due to CdS NPs strongly attached in TiO2/SiO2 surface, this result also demonstrating the CdS NPs good crystalline nature. The TiO2/SiO2–CdS nanocomposite, there is no other relevant peaks detected from the Fig. 2.
3.3 UV–Vis diffuse reflectance spectra
The DRS spectra of carried out to determine the optical parameter of the TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite as shown in the Fig. 3. Because of the presenting of TiO2 nanoparticle has a more extensive the optical properties of the light absorption compare with TiO2/SiO2 nanocomposites. Hence, this nanocomposite SiO2 and CdS NPs enhancing the absorption intensity of the visible light region somewhere is mediate for the essential band gap absorption of TiO2 nanoparticle resulting for the electron hole recombination of the valence band to the conduction band. The plot obtained the energy band gap calculated for the kubelka Munk function effects of energy band gap of the sample 3.18, 3.14, and 3.13 eV around ensuing to the TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite respectively [36, 37]. In the DRS plot, the TiO2/SiO2–CdS nanocomposite samples have been small absorption peak that of TiO2 NPs, however there are some red shift absorption available in the region of TiO2/SiO2–CdS nanocomposite are compared with the TiO2 NPs. Owing to this red shift adsorption edge may have been shifted chemical bonding between the TiO2/SiO2–CdS nanocomposite.
3.4 Surface morphology analysis
The surface morphology analysis was appeared in Fig. 4, TiO2 NPs, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite. The picture are complied with way of the nanoparticles are spherical shapes [36–39]. The surface morphology was the partial and did the significance of the arrangement of nanoparticles and the keeping surface nanostructure. It’s due to the spherical shape nanoparticles prepared by hydrothermal method, which could be displayed the highest photocatalytic activity. The exhibited a clear spherical shape morphology uniform particle size formed in Fig. 4b. Well-defined spherical structure of the nanocomposite prepared is supposed to have few structure defects. So it is clear formed to the nanocomposites for CdS is uniformly deposited on the surface morphology structure which is shown in Fig. 4c. In the TiO2/SiO2–CdS nanocomposites is annealing nature of spherical shape particle growth of porous structure is developed. Highly enhancing electron hole recombination centres which are catalytic performance of the dye molecules. This TiO2/SiO2–CdS nanocomposite materials evidently confirmed in EDAX spectra, we apparently confirmed that the Ti, Si, Cd, O and S shown in the Fig. 4d.
3.5 FT-IR spectra
The FT-IR spectra were investigated for the composition of TiO2 NPs, TiO2/SiO2, and TiO2/SiO2–CdS nanocomposite are shown in Fig. 5. There are exhibit peaks at 469 and 525 cm−1 are stretching vibration of Ti–O–T network. Its due to the presence of 3409 and 1725 cm−1, which might be assigned to the bending and stretching vibrations of the –OH groups and adsorbed for H2O molecule [32–35]. The FT-IR regions wave number at 1093 and 772 cm−1 are assigned to the symmetric and asymmetric stretching vibrations of Si–O–Si. Then the peaks appeared range of 911 and 993 cm−1 to the present Ti–O–Si observed vibration. All the bands also overlap with Si–O–H bonds and the peaks at 900 and 1368 cm−1 are attributed to the stretching vibrations of C–O respectively.
3.6 Evaluation of photocatalytic activity
The photocatalytic reaction was carried out initiated when TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite absorbed photon in direct sunlight or UV–Visible light. Catalytic degradation was carried out the dye solution stirred in a dark room for 30 min, to obtained from absorption and desorption equilibrium level. At once the catalyst results show the dye solution is a lightly stabled in the nanocomposite of photocatalyst which is shown in the Fig. 6. Hence, the absorption of light electron valance band (VB) to TiO2/SiO2–CdS nanocomposite promotes the conduction band (CB) is due to the formation of electron hole in the valence band (VB). The photon generated holes and fast recombination of electron have induced the oxidation reduction reaction was occurred. Rapid synthesis of composite materials, such as electron hole pair’s reproduction of electron dissipates in the heat producing slow down the photo catalytic dye degradation [6, 40–44]. This are degradation may due to adsorption and desorption processes dye on the surface. The catalyst observed for dye on UV–Visible light on (0.1%) of reaction was carried out any catalyst. Owing to this observation of reveal to the Visible light and catalyst are needed to the effective catalytic degradation of MB dye. The bare TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite were under the reaction carried out for 51, 74 and 85% methylene blue dye degradation respectively. This shows the perforation of TiO2/SiO2–CdS nanocomposite are effective, increasing highly enhance the catalytic degradation of dye.
The photocatalytic dye degradation of methylene blue was carried out the experiment optimum conditions for concentration of 10 mg/L−1 TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite dosage 0.1 g/L for irradiation of adsorption desorption equilibrium time at 60 min has shown in the Fig. 7. In the experiment catalytic degradation where pseudo first order kinetics rate constant (plots of −ln (C/C0) verses to time showed linear relationship were C is a concentration of MB dye [42, 43]. When the integration limit of C/C0 at t = 0 with C0 is the equilibrium concentration of bulk solution of methylene blue dye. When this following equation \(\ln (\frac{{{C}_{0}}}{C})\) = KApp t, KApp is the apparent first order kinetics of the rate constant (min−1). The degradation rate constant value for photocatalytic degradation of MB dye were determined TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite to be k = 0.8321, k = 1.0357 and k = 1.2464 min−1 respectively.
3.7 Mechanism of photocatalytic activity
The proposed mechanism of electron generated by sun light are transferred from conduction band of CdS to conduction band of TiO2/SiO2, whereas electron holes was transferred from valance band of TiO2/SiO2 to valance band of CdS active surface are shown in Fig. 8. This helps to hinder the recombination of photo generated electrons and holes and enhance the allegation, departure efficiency. The surrounding oxygen molecules adsorbed on the photocatalyst form superoxide anion radicals (·O2 −) due to their interaction with electrons in the conduction band of TiO2. The external, hydroxyl groups harvest extremely reactive hydroxyl (·OH) radicals by behave with holes in the valence band of CdS. The dye molecules are photo degrade by the reaction with both of them radicals (·OH and ·O2 −) [45]. For the much better memorandum of the underlying mechanism, Yang et al. [46] have monitored the formation of hydroxyl radicals (·OH) and superoxide radicals (·O2 −) during visible-light-induced photocatalytic degradation of methylene blue.
4 Conclusion
The nano sized TiO2, TiO2/SiO2 and TiO2/SiO2–CdS nanocomposite good excellent spherical structure and high surface area. The CdS NPs is doped in TiO2/SiO2 surface; the XRD spectra are crystalline nature. It’s due to significance of using solar energy as the irradiation source to improve the catalytic activity of TiO2 obviously by appropriately doping of TiO2/SiO2–CdS nanocomposite. The presentation of CdS NPs in TiO2/SiO2 is capable not only for reducing the photo-generated hole-electron recombination rate but also for utilizing solar energy sufficiently. Hence, it is confident to utilize CdS NPs composite with TiO2/SiO2 as an effective photocatalyst in the degradation of organic contaminates. These TiO2/SiO2–CdS nanocomposites have highly irradiation of photocatalytic degradation of methylene blue dye efficiency of (88%).
References
D.H. Wang, L. Jia, X.L. Wu, L.Q. Lu, A.W. Xu, Nanoscale 4, 576–584 (2012)
A.A. Ismail, D.W. Bahnemann, J. Mat. Chem. 21, 11686–11707 (2011)
X.Y. Zhang, H.P. Li, X.L. Cui, Y. Lin, J. Mat. Chem. 20, 2801–2806 (2010)
E. Pajootan, M. Arami, M. Rahimdokht, Ind. Eng. Chem. Res. 53, 16261–16269 (2014)
Q. Xiang, J. Yu, M. Jaroniec, Chem. Soc. Rev. 41, 782–796 (2012)
C. Lin, Y. Song, L. Caoc, S. Chen, Nanoscale 5, 4986–4992 (2013)
T. Naveen Reddy, J. Manna, R.K. Rana, ACS Appl. Mater. Interfaces 7, 19684–19690 (2015)
R. Bera, S. Kundu, A. Patra, ACS Appl. Mater. Interfaces 7, 13251–13259 (2015)
G.R. Xua, J.N. Wang, C.J. Li, Appl. Surf. Sci. 279, 103–108 (2013)
N. Pugazhenthiran, S. Ramkumar, P. Sathish Kumar, S. Anandan, Microporous Mesoporous Mater. 131, 170–176 (2010)
T. Ochiai, A. Fujishima, J. Photochem. Photobiol. C 13, 247–262 (2012)
Y. Liao, W. Que, Q. Jia, Y. He, J. Zhang, P. Zhong, J. Mater. Chem. 22, 7937–7944 (2012)
W. Guo, C. Xu, X. Wang, S. Wang, C. Pan, C. Lin, Z.L. Wang, J. Am. Chem. Soc. 134, 4437–4441 (2012)
G. Sahu, K. Wang, S.W. Gordon, W. Zhou, M.A. Tarr, RSC Adv. 2, 3791–3800 (2012)
J. Hyeok Park, S. Kim, A.J. Bard, Nano Lett. 6, 24–28 (2006)
X. Meng, D.W. Shin, S.M. Yu, J.H. Jung, H.I. Kim, H.M. Lee, Y.H. Han, V. Bhoraskarac, J. Beom Yoo, Cryst. Eng. Comm. 13, 3021–3029 (2011)
B.D.V. Bavykin, J.M. Friedrich, F.C. Walsh, Adv. Mater. 18, 2807–2824 (2006)
A. Pandikumar, S. Manonmani, R. Ramaraj, Catal. Sci. Technol. 2, 345–353 (2012)
A. Yanhui, J. Xu, S. Zhang, D. Fu, Appl. Surf. Sci. 256, 2754–2758 (2010)
K. Kawata, S.N. Gan, D.T.C. Ang, K.P. Sambasevam, S.W. Phang, N. Kuramoto, Poly. Compos. 34, 1884–1891 (2013)
A. Pandikumar, R. Ramaraj, J. Renew. Sustain. Energy 5, 043101 (2013)
H. Song, S. You, T. Chen, X. Jia, J. Mater. Sci. 26, 8442–8450 (2015)
N. Pugazhenthiran, S. Murugesan, S. Anandan, J. Hazard. Mater. 263, 541–549 (2013)
H. Zhao, L. Liu, J.M. Andinobc, Y. Li, J. Mater. Chem. A. 1, 8209–8216 (2013)
W.N. Wang, J. Park, P. Biswas, Catal. Sci. Technol. 1, 593–600 (2011)
P. Govindhan, C. Pragathiswaran, J. Mater. Sci. 27, 8778–8785 (2016)
S. Yana, B. Wanga, Y. Shib, F. Yanga, D. Hua, X. Xua, J. Wuaa, Appl. Surf. Sci. 285, 840–845 (2013)
J. Xu, H. Sang, X. Wang, K. Wanga, Dalton Trans. 44, 9528–9537 (2015)
N. Zhang, Y. Zhang, X. Pan, X. Fu, S. Liu, J.Y. Xu, J. Phys. Chem. C 115, 23501–23511 (2011)
X. Lia, P. Liua, Y. Maoa, M. Xinga, J. Zhanga, Appl. Catal. Environ. 164, 352–359 (2015)
S. Wang, M. Zhang, W. Zhang, ACS Catal. 1, 207–211 (2011)
M.R. Delsouz Khaki, B. Sajjadi, A.A. Abdul Raman, W.M.A. Wan Daud, S. Shmshirband, Measurement 77, 155–174 (2016)
Y. Liu, B. Zhang, L. Luo, X. Chen, Z. Wang, E. Wu, D. Su, W. Huang, Angew. Chem. Int. Ed. 54, 15260–15265 (2015)
T.T. Duong Vu, F. Mighri, A. Ajji, T.O. Do, Ind. Eng. Chem. Res. 53, 3888–3897 (2014)
J. Di, S. Li, Z. Zhao, Y. Huang, Y. Jia, H. Zheng, Chem. Eng. J. 281, 60–68 (2015)
X. Gou, Y. Cheng, B. Liu, B. Yang, X. Yan, Eur. J. Inorg. Chem. 13, 2222–2228 (2015)
T. Zhu, W.L. Ong, L. Zhu, G.W. Ho, Sci. Rep. 5, 10601 (2015) doi:10.1038/srep10601.
K. Ullah, S. Ye, Z. Lei, K.Y. Chob, W. Chun Oh, Catal. Sci. Technol. 5, 184–198 (2015)
M. Wang, J. Han, H. Xiong, R. Guo, Langmuir 31, 6220–6228 (2015)
G. Mishra, K.M. Parida, S.K. Singh, ACS Sustain. Chem. Eng. 3, 245–253 (2015)
V.R. De Mendonc, H.A.J.L. Mour, A.R. Malagutti, C. Ribeiro, Photochem. Photobiol. 90, 66–72 (2014)
B. Subash, B. Krishnakumar, M. Swaminathan, M. Shanthi, Spectrochim. Acta A 105, 314–319 (2013)
X. Pan, Y.J. Xu, J. Phys. Chem. C 119, 7184–7194 (2015)
N.K. Rao Eswar, P.C. Ramamurthy, G. Madras, Photochem. Photobiol. Sci. 14, 1227–1237 (2015)
L. Yuan, C. Wang, R. Cai, Y. Wang, G.J. Zhou, Appl. Phys. 114, 023512 (2013)
Y. Yang, G. Zhang, W.J. Xu, Colloid Interface Sci. 376, 217–223 (2012)
Acknowledgements
The author, P. Govindhan thanks the Branch Manager, Syndicate Bank, Matalampatti, Dharmapuri, sanctioning for education loan to conduct for their support to carry out this research work. C. Pragathiswaran belongs to research article express his gratitude to the Staff Members, Department of Chemistry, Periyar E.V.R. College (Autonomous), Triuchirappalli-23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Govindhan, P., Pragathiswaran, C. Enhanced photocatalytic activity of TiO2/SiO2–CdS nanocomposite under direct sunlight for degradation of methylene blue. J Mater Sci: Mater Electron 28, 5063–5069 (2017). https://doi.org/10.1007/s10854-016-6164-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6164-z