Abstract
The effect of Cr2O3 on the microstructure and dielectric properties of SnO2 varistors doped with Nb2O5 and CoO was investigated. The density of ceramic samples was calculated and found increases from 6.6819 to 6.8704 g cm−3. It is observed in this figure that, independent of the Cr2O3 concentration, no other crystalline phase besides SnO2 is observed indicating that the system is single phase within the detection precision of the X-ray diffraction. The average crystallite size of the prepared samples using was estimated Scherrer’s equation. A scanning electron microscope (SEM) was utilized in order to study the effect of variation of Cr2O3 content and its impact on the microstructure and electric properties. The obtained results show that, as Cr2O3 concentration increases the dielectric permittivity increases and ac resistivity decreases and then increases and takes it maximum resistivity at 0.5 mol%. The compositional dependence of the dielectric permittivity and ac resistivity has been explained in the light of the SEM micrographs. The obtained results show also that the sample of 0.5 mol% of Cr2O3 has highest resistivity and consequently low dielectric loss. So, it can be used in some technological applications such as transformer and inductor cores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Varistors are very important materials used as voltage pulse suppers and over voltage protectors in electronic systems and industrial equipment. SnO2 ceramics are n-type semi-conductors with native oxygen vacancies compensated by electrons, it similar to ZnO, but it does not densify when sintered without additives. Dense SnO2-based ceramics can be achieved by introducing dopants such as CoO and MnO2 or by hot isostatic pressing [1–3]. SnO2 has very low densification rate due to its high surface diffusion at low temperatures and high pressure at high temperatures. Also, the addition of CoO and MnO2 to SnO2 produces high densification allowing for the development of other electronic devices [4–6]. The nonlinear electrical properties of SnO2 ceramics and other systems have been reported by many authors [7–12]. On the other hand, the dopants such as Nb2O5 and Ta2O5 play an important role to enhance the degree of nonlinearity, which can be also described as varistor forming oxide as in the field of ZnO varistors [13, 14].
In SnO2 ceramic system, Nb increases the electrical conductivity of grains and Co is extremely active in the promotion of Sn oxide densification. Addition of trivalent transition oxides such as Fe2O3, Cr2O3 and rare earth oxides improves significantly the electrical characteristics of SnO2-based varistors [15–22]. In the previous works, the effects of CuO on the grain size, density, nonlinearity, and dielectric properties of the SnO2–Ni2O3–Nb2O5 and SnO2–Co2O3–Ta2O5 varistors were investigated [23, 24]. They shows that the breakdown electric field of the SnO2 varistors decreases with increasing CuO content and the nonlinear coefficient possesses a peak value of 31 and the leakage current density has the minimum value of 2 l A/cm2 at 0.2 mol% CuO sintered at 1300 °C. So, 0.2 mol% is the optimum CuO content for SnO2 varistors, and 1300 °C is the best sintering temperature for this system [25].
In the present work we study the effect of Cr2O3 concentration on the structure and dielectric properties of nanocrystalline SnO2 varistors doped with Nb2O5 and CoO to determine the optimum concentration of Cr2O3 in this varistor for the technological applications.
2 Experimental
Samples with chemical composition ((98.95 − x) SnO2 + 0.05Nb2O5 + CoO + xCr2O3) mol% (x = 0, 0.1, 0.3, 0.5), namely C1, C2, C3 and C4 respectively were prepared using a conventional ceramic method. All the oxides were raw powders of analytical grade. At beginning, the raw powders were mixed in deionized water and ball-milled in polyethylene bottle for 4 h with 0.3 wt% of PVA as binder and highly wear-resistant ZrO2 balls as grinding media. Subsequently, the obtained slurries were dried at 210 °C. After drying, the powder chunks were crushed into fine powders and sieved. Discs of 2 cm diameter and 0.2 cm thickness were obtained by a semi-dry press method under 70 KN. Finally the samples were sintered at 1300 °C for 2 h. The crystalline phases were identified by (XRD, using Philips apparatus type 170, Cu K α and λ = 1.5418 Å) through a continuous scan mode with speed of 10°/min. The microstructure was examined on the fresh fracture surfaces of the samples via a scanning electron microscope (SEM Model Quanta 250 FEG) attached with EDAX unit, with accelerating voltage 30 kV, magnification 14× up to 106 and resolution for Gun 1n. To measure the dielectric properties, silver electrodes were placed on both surfaces of the sintered disks. A programmable automatic R–C–L meter PM 6304 was used for the measurements of ac resistance and capacitance. From the measured values of ac conductivity and the dielectric constant at all frequency from 50 Hz to 5 MHz were calculated at constant temperature.
3 Results and discussion
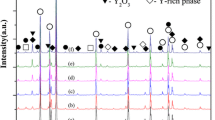
The density and water absorption of the prepared samples were calculated using Archimedes method and the obtained shown in Fig. 1. This figure shows the addition of Cr2O3 results in an increase in the bulk density of the samples from 6.6819 to 6.8704 g cm−3. The increase of the density may be attributed to the difference between the molecular weight SnO2 and Cr2O3. Also, when Cr3+ cation replaces Sn+4 in the lattice, it acts as an electron acceptor leading to the formation of oxygen vacancies, and consequently increasing the solid state diffusion rate. This diffusion promotes large densification and therefore the density increases. Water absorption of the material is defined as the amount of water absorbed by a material after immersion in water for a certain time. Figure 1 show also that, as density of the samples increases the water absorption decreases due to the decreases in the porosity of the samples. The calculated density of SnO2 varistor sample was compared with the theoretical density of SnO2 (6.95 g cm−3), this prefigures that SnO2-based varistors are possible candidates for many dense industrial applications. Figure 2 illustrates the XRD of the as-prepared SnO2-based varistor ceramics with different amounts of Cr2O3 doping. It was observed from the X-ray diffraction pattern that there is no appearance of any secondary phases produced by dopants in all samples except the SnO2 phase (JCPDS card No. 77-0451). The absence of any diffraction line, Nb2O5, CoO and Cr2O3 is attributed to the complete dissolution of these dopants oxides in the lattice of SnO2 and forming solid solution or the existence of Nb2O5, CoO and Cr2O3 in extremely small size that could not be detected by XRD. Similar results were obtained in a previous work [25].
The average crystallite size (t) of the prepared samples was estimated using Scherrer’s equation [26],
where β is the peak width at half maximum, λ is wavelength and θ corresponds to the peak position. The obtained results shown in Fig. 3 indicate that the average crystallite size increase as Cr2O3 mol% increases.
SEM of the samples is shown in Fig. 4. The microstructure of the prepared ceramics consists of SnO2 grains doped with Cr2O3, CoO and Nb2O5. It reveals that the surfaces of all as prepared samples possess a plain texture with the expected clear grain boundary for ceramic materials. A scanning electron microscope (SEM) was utilized in order to study the effect of variation of Cr2O3 content and its impact on the microstructure and electric properties, these microstructures are not greatly different with varistor ceramics doped with Er, Y and Dy oxides as reported previously [27–29]. It is clearly evident that by increasing the dopant level grain size becomes larger that is in good agreement with the calculated values using Scherrer’s equation. EDAX of C4 shows the distribution of Co, Cr and Nb between SnO2 and SnO2 grains as shown in Fig. 5.
Figure 6 shows the variation of the dielectric permittivity as a function of frequency for all samples. Obviously, the dielectric constant shows a decreasing trend for all the samples. The decrease is rapid at lower frequency and slower and stable at higher frequency. At low frequency range (50–1 kHz) the decrease of ε′ value is more pronounced than at higher frequency due to the movement of charge carriers trapped at interfacial region which is caused by inhomogeneous dielectric structure. At high frequency, the dominant mechanism contributing to dielectric constant is the hopping mechanism in their interstice under the influence of alternating field. The frequency of hopping between ions could not follow the frequency of applied field and hence it lags behind, therefore the values of dielectric constant decreases at higher frequency [30]. Figure 7 shows the variation of the dielectric loss ε″ as a function of frequency for all samples. The dielectric loss shows a decreasing trend with the increase of the frequency for all the samples due to the decreasing in the electrical resistivity.
The relation between ac conductivity and frequency for different mixes are shown in Fig. 8. Figure 8 shows that, the electrical conductivity increases with increasing the frequency at room temperature. The conductivity increased slowly in low frequency region but sharply in high frequency region. This behavior can be explained in the light of hopping model of charges [31]. According to this model, the increase in frequency of the applied field enhances the hopping of charges between the charge carriers Sn4+ and Sn2+ and hence the conductivity increases. Moreover, the increasing frequency of the applied field liberates the charges trapped in deep traps.
The compositional dependence of the dielectric constant, dielectric loss and ac resistivity is shown in Table 1. Table 1 shows that, as Cr2O3 concentration increases the dielectric permittivity increases and ac resistivity decreases and then increases and takes it maximum resistivity at 0.5 mol%. When a Cr3+ cation replaces Sn+4 in the lattice it acts as an electron acceptor leading an increasing in the dielectric permittivity, ac conductivity and consequently the resistivity decreases. The increases in the resistivity at x = 0.5 mol% can be explained in the light of SEM micrographs. The increase in the resistivity at x = 0.5 mol% is attributed to the segregation of ions at the grain boundaries as shown in the SEM micrographs which acts as scattering centers that oppose the electron flow [32] and consequently increases the electrical resistivity for the investigated system which reduces the occurrence of the eddy currents. The samples also show high nonlinear coefficient in the range of 29–41.
The electrical resistivity of the varistor system can be improved with the addition of pentavalent ions such as Sb2O5, Nb2O5 and V2O5 which act as electron donors to the crystal lattice according to the following equation [33–35]
Trivalent cations such as chromium, ytterbium and scandium that act as acceptors of electrons are added to SnO2 crystal lattice which used to improve the varistor system [35–39]. There are change in the oxidation state of CoO and Cr2O3 by increasing temperature, according to the following reactions: [40]
when a Cr3+ cation replaces Sn+4 in the lattice, it acts as an electron acceptor leading to the formation of oxygen vacancies, thus increasing the solid state diffusion rate through the SnO2 lattice, according to the equations below: [41]
The segregation of these ions in the grain boundary increase the resistivity values and causes the improvement of nonlinear coefficient due to the higher absorption of electron acceptor species on the grain boundary surface, increasing the barrier height potential and decreasing the conductivity, as demonstrated in the above Eqs. (5–7) [42–44]. Bueno et al. [45] reported that, the mechanisms responsible for the formation of the potential barrier are related to the presence of oxygen species adsorbed on the grain boundary region such as O− and O−, which donate electrons for the oxygen, providing the formation of a region with negative charge density. CoO and Cr2O3 are also responsible for the formation of barrier in SnO2 varistors. The obtained results show that the sample of 0.5 mol% of Cr2O3 has highest resistivity, low dielectric loss and consequently reduces the energy loss as heat. Accordingly they can be used in many applications such as transformer cores and memory cores.
4 Conclusions
The substitution by Cr2O3 improves the microstructure of SnO2 varistors where the density increases from 6.6819 to 7.2803 g cm−3. SEM of the prepared samples sintered confirming the presence of particles of non-uniform structure having single almost spherical particles of small diameter ranged from 51 to 83 nm. The dielectric permittivity, dielectric loss and ac resistivity show a decreasing trend with the frequency for all the prepared samples. The obtained results show that, as Cr2O3 concentration increases the dielectric permittivity increases and ac resistivity decreases and then increases and takes it maximum resistivity at 0.5 mol%. The compositional dependence of the dielectric constant and ac resistivity has been explained in the light of SEM micrographs. The obtained results show also that the sample of 0.5 mol% of Cr2O3 has highest resistivity and consequently low dielectric loss. So, it can be used in some technological applications such as transformer and inductor cores.
References
M.S. Castro, C.M. Aldao, Characterization of SnO2-varistors with different additives. J. Eur. Ceram. Soc. 18, 2233–2239 (1998)
A.N. Fauzana, B.Z. Azmi, M.G.M. Sabri, W.R. Wan, Abdullah & M. Hashim, Microstructural and nonlinear electrical properties of ZnO ceramics with small amount of MnO2 dopant. Sains Malays. 42(8), 1139–1144 (2013)
S.J. Park, K. Hirota, H. Yamamura, Densification of nonadditive SnO2 by hot isostatic pressing. Ceram. Int. 10(3), 115–116 (1984)
S.A. Pianaro, P.R. Bueno, E. Longo, J.A. Varela, A new SnO 2-based varistor system. J. Mater. Sci. Lett. 14, 692 (1995)
J.A. Varela, J.A. Cerri, E.R. Leite, E. Longo, M. Shamsuzzoha, R.C. Bradt, Microstructural evolution during sintering of CoO doped SnO2 ceramics. Ceram. Int. 25, 253–256 (1999)
A.C. Antunes, S.R.M. Antunes, S.A. Pianaro, M.R. Rocha, E. Longo, J.A. Varela, Nonlinear electrical behaviour of the SnO2.CoO.Ta2O5 system. J. Mater. Sci. Lett. 17, 577 (1998)
G.M.M.M. Lustosa, J.P.C. Costa, L.A. Perazolli, B.D. Stojanovic, M.A. Zaghete, Potential barrier of (Zn, Nb)SnO2-films induced by microwave thermal diffusion of Cr3+ for low-voltage varistor. J. Am. Ceram. Soc. 99(1), 152–157 (2016)
G.M.M.M. Lustosa, J.P.C. Costa, L.A. Perazolli, B.D. Stojanovic, M.A. Zaghete, Electrophoretic deposition of (Zn, Nb)SnO2-films varistor superficially modified with Cr3+. J. Euro. Ceram. Soc. 35, 2083–2089 (2015)
S.A. Pianaro, P.R. Bueno, P. Olivi, E. Longo, J.A. Varela, Electrical properties of the SnO2-based varistor. J. Mater. Sci.: Mater. Electron. 9, 158 (1998)
A.B. Glot, R. Bulpett, A.I. Ivon, P.M. Gallegos-Acevedo, Electrical properties of SnO2 ceramics for low voltage varistors. Phys. B 457, 108–112 (2015)
W. Yongjun, W. Jinfeng, C. Hongcun, Z. Weilie, Z. Peilin, D. Huomin, Z. Lianyi, Electrical properties of SnO2–ZnO–Nb2O5 varistor system. J. Phys. D 33, 96 (2000)
Z. Peng, R. Gao, F. Jiang, J. He, X. Fu, Microstructure and nonohmic properties of SnO2–Ta2O5–TiO2 varistor system doped with CuO. Int. J. Mod. Phys. B 28, 1450085 (2014)
P.R. Bueno, J.A. Varela, E. Longo, SnO2, ZnO and related polycrystalline compound semiconductor. J. Eur. Ceram. Soc. 28, 505–529 (2008)
D.R. Clarke, Varistor ceramics. J. Am. Ceram. Soc. 82, 485–502 (1999)
R. Parra, J.A. Varela, C.M. Aldao, M.S. Castro, Electrical and microstructural properties of (Zn, Nb, Fe)-doped SnO2 varistor systems. Ceram. Int. 31(5), 737–742 (2005)
S.A. Pianaro, P.R. Bueno, E. Longo, J.A. Varela, Microstructure and electric properties of a SnO2 based varistor. Ceram. Int. 25(11), 1–6 (1999)
J.F. Wang, W.B. Su, H.C. Chen, W.X. Wang, G.Z. Zang, (Pr Co, Nb)-doped varistor ceramics. J. Am. Ceram. Soc. 88(2), 331–334 (2005)
I.P. Silva, A.Z. Simoes, F.M. Filho, E. Longo, J.A. Varela, L. Perazolli, Dependence of La2O3 content on the non-linear electrical behavior of ZnO, CoO and Ta2O5 doped SnO2 varistors. Mater. Lett. 61, 2121–2125 (2007)
M.M. Oliveira, P.R. Bueno, M.R. Cassia-Santos, E. Longo, J.A. Varela, Sensitivity of SnO2 nonohmic behavior to the sintering process and to the addition of La2O3. J. Eur. Ceram. Soc. 21, 1179–1185 (2001)
M.M. Oliveira, P.C. Soares Jr., P.R. Bueno, E.R. Leite, E. Longo, J.A. Varela, Grain-boundary segregation and precipitates in La2O3 and Pr2O3 doped SnO2- CoO-based varistors. J. Eur. Ceram. Soc. 23, 1875–1880 (2003)
R. Parra, Y. Maniette, J.A. Varela, M.S. Castro, The influence of yttrium on a typical SnO2 varistor system: microstructural and electrical features. Mater. Chem. Phys. 94, 347–352 (2005)
P. Qi, J.F. Wang, W.B. Su, H.C. Chen, G.Z. Zhang, C.M. Wang, B.Q. Ming, (Er Co, Nb)-doped SnO2 varistor ceramics. Mater. Chem. Phys. 92, 578–584 (2005)
W.X. Wang, J.F. Wang, H.C. Chen, W.B. Su, G.Z. Zang, Electrical nonlinearity of (Cu, Ni, Nb)-doped SnO2 varistors system. Mater. Sci. Eng., B 99, 457 (2003)
C.M. Wang, J.F. Wang, H.C. Chen, W.B. Su, G.Z. Zang, P. Qi, M.L. Zhao, Mater. Sci. Eng., B 116, 54 (2005)
G. Hu, J. Zhu, H. Yang, Effect of CuO addition on the microstructure and electrical properties of SnO2-based varistor. J. Mater. Sci.: Mater. Electron. 24, 2944–2949 (2013)
W.D. Kigery, H.K. Bowen, D.R. Uhlmann, Introduction of Ceramics (Wiley, NewYork, 1975), p. 458
C.W. Nahm, Mater. Lett. 59, 2097–2100 (2005)
J.C. Wurst, J.A. Nelson, J. Am. Ceram. Soc. 55, 109–111 (1972)
M. Mukae, K. Tsuda, I. Nagasawa, J. Appl. Phys. 50, 4475–4476 (1979)
G.B. Kumar, S. Buddhudu, Optical, thermal and dielectric properties of Bi4(TiO4) ceramic powders. Ceram. Int. 36, 1857–1861 (2010)
N. Rezlescu, E. Rezlescu, Phys. Status Solidi A 59, 323 (1980)
N. Rezlescu, E. Rezlescu, C. Pasnicu, M.L. Craus, J. Magn. Magn. Mater. 136, 319 (1994)
I.O. Mazalli, W.C. Las, M. Cilense, The effect of preparation method and Sb content on SnO2–CuO sintering. J. Mater. Sci. 38, 3325–3330 (2003)
S.A. Pianaro, P.R. Bueno, P. Olivi, E. Longo, J.A. Varela, Electrical properties of the SnO2-based varistor. J. Mater. Sci.: Mater. Electron. 9, 159–165 (1998)
A.V. Gaponov, A.B. Glot, A.I. Ivon, A.M. Chack, G. Jimenes-Santana, Varistor and humidity-sensitive properties of SnO2–Co3O4–Nb2O5–Cr2O3 ceramics with V2O5 addition. Mater. Sci. Eng., B 459, 76–84 (2007)
W.X. Wang, J.F. Wang, H.C. Chen, W.B. Su, G.Z. Zang, Effects of Cr2O3 on the properties of (Co, Nb)-doped SnO2 varistors. Mater. Sci. Eng., B 9, 470–474 (2003)
J.A.A. Martínez, M.I.P. Canul, M.B. Hernández, A.B. Glot, E. Rodrígez, L.G. Ortiz, Effect of Cr2O3 on the microstructure and non-ohmic properties of (Co, Sb)-doped SnO2 varistor. Revista Mexicana de Física 59, 6–9 (2013)
W.X. Wang, J.F. Wang, H.C. Chen, W.B. Su, B. Jiang, G.Z. Zang, C.M.P. Wang, Qi, Varistor properties of Sc2O3-doped Sn.Co.Nb ceramics. Ceram. Int. 31, 287–291 (2005)
D.R. Leite, W.C. Las, M.A. Zaghete, M. Cilense, J.A. Varela, The effect of Cr concentration and preparation method on the microstructure and electrical characterization of SnO2-based ceramics. Mater. Res. 6, 457–461 (2003)
W.C. Ming, W.J. Feng, C.H. Cun, S.W. Bin, Z.G. Zhong, Q. Peng, Effect of Er2O3 an electrical properties of SnO2–CoO–Ta2O5 varistor system. Chin. Phys. Lett. 21(4), 716 (2004)
S.A. Pianaro, P.R. Bueno, E. Longo, J.A. Varela, J. Mater. Sci. Lett. 14, 692 (1995)
W.K. Bacelar, P.R. Bueno, E.R. Leite, E. Longo, J.A. Varela, How Cr2O3 influences the microstructure and nonohmic features of the SnO2 (Cox, Mn1−x)O-based varistor system. J. Eur. Ceram. Soc. 26, 1221–1229 (2006)
G. Brankovic, Z. Brankovic, M.R. Bavolos, M. Cilense, J.A. Varela, Influence of the common varistor dopants (CoO, Cr2O3 and Nb2O5) on the structural properties of SnO2 ceramics. Mater. Charact. 52, 243–251 (2004)
W.X. Wang, J.F. Wang, H.C. Chen, W.B. Su, B. Jiang, G.Z. Zang, C.M. Wang, P. Qi, Varistor properties of Sc2O3-doped Sn.Co.Nb ceramics. Ceram. Int. 31, 287–291 (2005)
P.R. Bueno, S.A. Pianaro, E.C. Pereira, L.O.S. Bulhoes, E. Longo, J.A. Varela, J. Appl. Phys. 84, 3700 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desouky, O.A., Rady, K.E. Synthesis, structure and dielectric properties of nanocrystalline SnO2–CoO–Nb2O5 varistor doped with Cr2O3 . J Mater Sci: Mater Electron 28, 4197–4203 (2017). https://doi.org/10.1007/s10854-016-6041-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6041-9